1000/1000
Hot
Most Recent

The biodegradable term refers to a biologically assisted degradation process. A biodegradable polymer is a polymer susceptible to degradation by biological activity, accompanied by a lowering of its molar mass. There is a justified need to investigate biodegradable polymers in form of thin films because compared with those bulk, which has a long and comprehensive history on the degradation subject, there is still insufficient and fragmented understanding about the degradation of thin polymeric films, with research limited in general to very specific cases. Our efforts have been focused on on centralizing the comprehensive literature studies about stability and degradation of different polymeric coatings, which were thoroughly evaluated by polymer mass loss measurements, electrochemical investigations and/or surface. A briefly description of the factors affecting degradation, testing methods and applications of biodegradable polymers coatings was completed.
Undoubtedly, biodegradable polymers have a major role in biomaterials and medical devices (MD) functionalization fields, due to their capability for property tailoring and to completely degrade in time under physiological conditions [1]. It is well known that biomaterials interact with biological systems through their surfaces [2][3] and so that it is of great importance to control or tune the surface properties of MD, helping them integrate safely and easily into the host tissues [4].
In this entry, several advantages could be obtained by MD functionalization with polymer-based thin films due to remarkable properties of biodegradable polymers. Beside biocompatibility, they can also incorporate drugs by achieving a predetermined drug release profiles at a desired site of action meanwhile being easily eliminated by the body or even replaced in time by tissues [1].
The potential of surface functionalization with polymeric coatings (PC) (Figure 1) could solve the critical problems of used polymer amount supply associated with processing time. Moreover, thin film deposition techniques are designed to ensure the MD functionalization by improving the surface features with a newly formed polymeric platform. Concretely, polymeric thin films, present an amazing versatility in the chemical groups which can help control biomaterial – tissue interactions and also possess the required mechanical properties due to substrate [5]. As mentioned above, the relative easy processing time is another reason for the extensive interest in polymeric thin films. Surfaces can be coated with polymers using simple techniques such as: Langmuir-Blodgett deposition [6], spin – coating [7], sputtering [8], chemical vapor deposition [9][10], electrochemical deposition [11], spray – coating [12] or advanced laser techniques (e.g. matrix assisted pulsed laser evaporation [13]), Chemical grafting [14], self – assembled monolayers [15], surface - tethered polymers (polymer brushes) [16], dip-coating [17][18], electrophoretic deposition method [19] or multilayer [20][21].
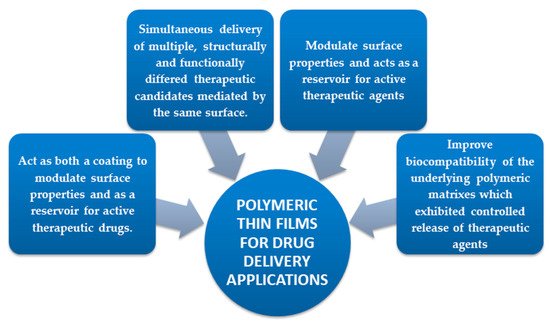
Figure 1. Benefits of surface functionalization with biopolymeric coatings.
Each deposition technique may show better results in a given situation and so that the choice of the proper coating fabrication method should be therefore determined based on the desired medical application, polymers’ workability and its physico-chemical properties [22]. An excellent option for the surface modification of metallic implants is the tailor-made coating (for accelerated tissue regeneration, with antibacterial properties and controlled release), making them more affordable and reducing or even eliminating the need for further surgical revisions [23]. Thin films also present advantages over bulk polymers due to their large surface-to-volume ratios, being suitable for applications requiring enhanced surface interactions. Another benefit of coatings over raw materials is the achievement of application-specific properties that are unattainable in the case of uncoated material or in the raw starting material used to be applied for surface functionalization.
With the pursuit that PC to be successfully integrated as functionalized MD , a critical discussion element of debate is the requirement of corroboration and analysis of polymeric thin films surface properties (such as morphology, micro- and/or nano- scale topography, chemical structure, and composition), with the dynamic phenomena taking place at interfaces (e.g. adsorption, modification, or wetting)) [24]. Each of these properties needs to be optimized for the achievement of a specific application. The control of the film properties requires tuning of the deposition parameters, which in return requires a thorough understanding of the underlying mechanisms of deposition and not only (e.g. type of polymer deposited and also on the interactions among process parameters and material parameters) [25].
At the same time, the next step toward more effectively designing - devices based on biodegradable polymers as coating materials is to gain a better understanding of the degradation behavior mechanisms and a deeper knowledge of structure–property relationships.
The development of polymeric functionalized coatings is in close connection with the degradation process. Although in its infancy, the study of the dynamic changes which occurs in a biodegradable polymeric thin film, can be extremely effective in predicting the body’s physiological regulation mechanisms and future performance of designed implantable medical devices.
This review is dedicated to the degradation mechanisms which occur in polymeric functionalized thin films with particular highlights on the correlation between raw polymer and the deposition techniques that allow to controlling and tuning of polymer properties and thus the functionality.
A digital search was performed for the period 2000-2021, using Web of Science (http://apps.webofknowledge.com) and following the criteria described in Figure 2.
There is still poor and fragmented understanding of the polymeric thin films degradation behavior as according Web of Science Core Collection (the research papers were limited to only 99 results). Comparatively, in the same period, 10,031 manuscripts on the polymeric thin films subject emphasize the advantages of applying polymer-based thin layers for medical devices functionalization. Search terms were put in double-quotes to restrict the search result to the specific phrases. The search field was specified to search only abstracts, title, keywords; and was restricted to only ISI journal articles. This statistic fully justifies the need to investigate biodegradable polymers in form of thin films because compared with those bulk (to which are attributed over 17,385 manuscripts), having a long and comprehensive history on the degradation approached subject of this review.
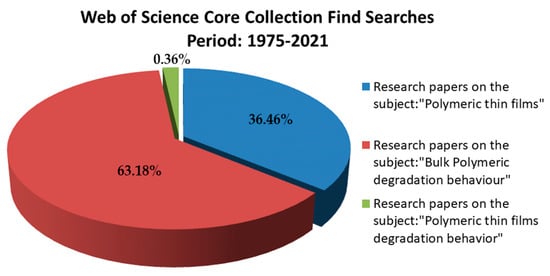
Figure 2. Current state of relevant publications available in the reviewed research field.
The difficulty with respect to the degradation behavior of biopolymers in form of coatings can be also related to the amount of an active substance (e.g. drugs, natural antimicrobial agents, etc.) that can be incorporated into the polymeric matrix and on the statistical process of synthesizing reproducible thin films. Another reasons for why degradation in polymer films is incompletely understood can be the large number of available biopolymers, extensive applications and compositions of thin polymeric films. Moreover, the diversity of potential substrates, numerous deposition methods, range of thicknesses that are possible, all these factors lead to a wide range of possible combinations to consider. In order to predict the functionalisation and performance of MD based on PC there is still place for studying the proposed subject.
Research efforts should be further directed toward improving and controlling biopolymers physico - chemical properties for long term in order to obtain sustainable coatings for drug delivery applications. Even better, as a perspective, composite personalized coating can be tuned according to the patient needs offering the opportunity for operability in the biopolymer composition and properties.
In this review is given a briefly introduction and classification of biodegradable polymers used as coatings, together with the degradation mechanisms and the factors affecting the process, as well as the testing methods and applications of biodegradable polymers coatings for drug delivery. Furthermore, current research approaches and future perspectives in the application of controlled degradation processes as alternative and viable routes toward enhanced polymer based coatings degradation and fictionalization is presented.
Taking into account that degradation process and biodegradable polymers are defined in a variety of ways in the literature it is fully justified the need of a short section regarding the used terminology. In this review, we have adopted the definitions as listed in the IUPAC Compendium of Chemical Terminology [26].
The biodegradable term refers to a biologically assisted degradation process. Concretely, according to IUPAC Compendium of Chemical Terminology [26], a biodegradable polymer is a polymer susceptible to degradation by biological activity, with the degradation accompanied by a lowering of its molar mass. Also here it is explained that a polymer degradation is represented by the chemical changes which appears in a polymeric material that usually result in undesirable changes or even deterioration in the useful properties of the material. The fragmentation means the breakdown of a polymeric biomaterial to particles regardless of the mechanism. There are illustrated the specific cases when degradation process is accompanied by a decrease in molar mass (e.g. vinyl polymers, polyamides) and the situations when degradation means changes in chemical structure (e.g. polymers with aromatic rings in the main chain). It can also be accompanied by crosslinking [26].
The degradation term is explained as a breakdown of a substance catalyzed by enzymes in vitro or in vivo [26]. Here, some other terms like bulk degradation, disintegration, dissolution, erosion, fragmentation, enzymatic or hydrolytic degradation process need to be clarified. Concretely, bulk degradation term refers to a faster degradation inside than at the surface of a polymeric biomaterial. The disintegration process is related to the particles fragmentation to an acceptable size (depending on the required application). Another discussion interferes in clarifying the dissolution term which is attributed to a solution of macromolecules constituting a polymeric biomaterial in a liquid medium and erosion which refers to a faster alteration at the surface than inside. At the same time we consider it necessary to define enzymatic degradation as degradation caused by the catalytic action of enzymes under abiotic experimental conditions meanwhile hydrolytic degradation is defined as degradation identified as resulting from hydrolytic cleavage of macromolecules. The term bioreactor is associated to an apparatus used to carry out any kind of bioprocess (examples include fermenter or enzyme reactor).
Definition, standard, and testing protocol for above mentioned terms are also reported in Ref. [27].
In order to be used in medical applications, a biodegradable polymer must fulfill certain criteria [28] such as:
- Non-toxic response after implantation in the body
- Reasonable shelf life
- Non-toxic degradation products able to get metabolized and easily eliminated from the body;
- The degradation time should match the therapy process time (e.g. healing, regeneration or treatment)
- Appropriate mechanical properties for the desired application and the inherence variation in mechanical properties that occurs with the degradation, compatible with the healing or regeneration process.
- Appropriate processability in order to tailor the mechanical properties of MD in correlation with the intended application.
The biopolymer based coatings designed for controlled release has the advantage of being able to maximizethe therapeutic benefit as they no require replacement or further manipulation and can degrade into non-toxic and soluble components [28].
Moreover, thin films term refers to layers of material (monolayer) ranging from nanometers to several micrometers in thickness.
There is another difference to be done between a thick and a thin film. Concretely, a thick film will typically have a thickness in the range 10 –25 μm (surface layers thicker than 1 micron are classified as coating) meanwhile thin films are usually surface layers with a sub-micron thickness (<500 nm) [29]. Furthermore, thin films deposition deals with the surface overlay with distinct atoms or molecules, while surface coating involves depositing particles [30]. However, the real difference between thin and thick films is more than their relative thickness, is the way in which they are synthetized [31]. Thin films are often deposited using vacuum techniques such as sputtering and molecular beam epitaxy meanwhile thick films are deposited from a solution or paste, which must be dried and then often sintered to produce the final coating [30].
Biopolymers present different degradation rates within the organism and therefore polymer selection can be tailored so as to achieve the desired release rates. Whatever the application or the desired degradation kinetics, it is essential to understand the degradation mechanisms in order to be able to define or guarantee either the stability and/or the controlled degradation rate.
In the following, we will refer only to the degradation process (which does not imply the microorganisms action) by dynamic simulations after the immersion of functionalized implants (thin polymeric coatings) in simulated body fluids ( SBF) or phosphate buffered saline (PBS) solutions. The degradable polymeric delivery systems described in this review will comprise polymeric systems that are degraded by surface or bulk erosion by hydrolysis or solubilization and their evolution in time.
One should mention that biodegradation actually involves two complementary processes: degradation (refers to the chain scission of polymer chains cleaved into oligomers and monomers) and erosion (represents the loss of material due to monomers and oligomers leaving the polymer), respectively. The process of polymers degradation can take place passively by hydrolysis or actively by enzymatic reaction so in this context we made the classification taking into account the way in which it biodegradable polymers degrades.
Biodegradable polymers can be classified also according to their origin as natural or synthetic polymers. Most of natural-based polymers are completely biodegradable while the synthetic ones and their blends do not degrade completely. Both of these are in the same time subdivided into different classes based on the main linkages present in their structure.
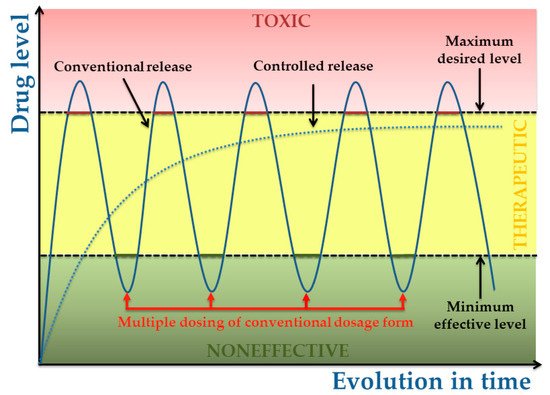
Figure 3. Schematic representation of biodegradable biopolymers classification.
Based on their degradation mechanisms, biopolymers can be next classified into bulk (homogeneous) and surface (heterogeneous) eroding biomaterials (Figure 4.) [32].
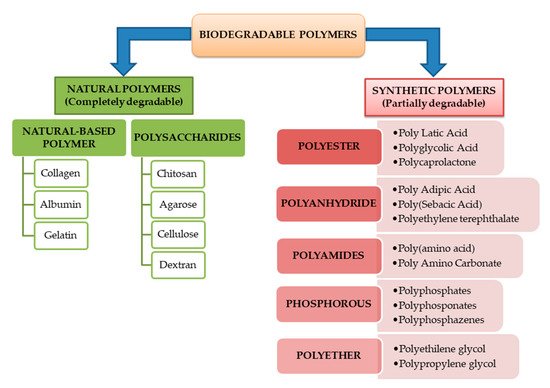
Figure 4. Schematic Representation of Surface Erosion versus Bulk Erosion
In the case of an ideal bulk erosion, the erosion rate depends on the total amount of the material (biomaterial is lost from the entire polymer volume at the same time due to water penetrating the bulk while remaining a constant size during the degradation process). Some biopolymers that exhibit this characteristic bulk erosion degradation mechanism are the biodegradable polyesters: poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), polyglycolic acid (PGA) and poly-L-lactic acid (PLLA). The erosion time of these polymers, widely used in drug delivery applications, varies from a few months to several years (depending on the monomers used and their molecular weights) [33].
In ideal surface erosion, the erosion rate will be proportional to the surface area. Concretely, polymers surface eroding become smaller while keeping their original geometric shape during the degradation process (biomaterial is lost only from the polymer matrix surface). Some characteristic examples of polymers which exhibit characteristic surface erosion degradation mechanism are polyanhydrides as e.g. poly(sebacic acid) (PSA), poly[1,3-bis (p-carboxyphenoxy) propane-sebacic acid] (PCPP:SA), poly(1,3-bis(p-carboxyphenoxy) propane (PCPP) and poly(1-6-bis(p-carboxy phenxoy)hexane) (PCPH). These are generally hydrophobic polymers wherein water cannot penetrate easily into the bulk. The erosion time in this case can vary from few days to several years [34]