1000/1000
Hot
Most Recent

Sperm plasma membrane lipids are essential for the function and integrity of mammalian spermatozoa. Various lipid types are involved in each key step within the fertilization process in their own yet coordinated way. For instance, loss of asymmetric transbilayer distribution and the substantial loss of cholesterol and phospholipid occur during capacitation and acrosome reaction (AR). The balance between lipid metabolism is tightly regulated to ensure physiological cellular processes, especially referring to crucial steps such as sperm motility, capacitation, acrosome reaction or fusion. At the same time, it has been shown that male reproductive function depends on the homeostasis of sperm lipids.
Spermatozoa are characterized as specified haploid cell types that lack most organelles and DNA transcription, resulting in the arrest of protein synthesis and vesicle transport [1]. Spermatozoa possess glycolytic and respiratory capacities, which are predominantly related to the need to maintain cell motility [2]. Additionally, as research progressed, spermatozoa were found to feature lipid synthesis, such as de novo synthesis of phosphatidylcholine [2][3]. The organization of eukaryotic cells depends to a large extent on the structure and function of membranes, based upon the intrinsic properties of membrane lipid components [4]. Spermatozoa lack typical membrane-structured organelles such as the endoplasmic reticulum and Golgi apparatus. The main membrane structures are used to separate cellular regions, such as plasma membrane, outer and inner acrosomal membrane and nuclear envelope (reviewed in [1]). The lipid distribution of spermatozoa can be described with a number of features, including (1) the lipid compositions of the sperm head and tail are different [5]; (2) the plasma membrane of the spermatozoa head shows lateral heterogeneity of surface molecular topology [6][7]; and (3) lipids are also characterized by asymmetric distribution in the membrane bilayer structure [1].
Alterations of lipid components in membranes are usually related to their physiological requirements. For example, the release, modification and adsorption of lipids occur during the transfer of sperm cells through the epididymis [1][8]. Extracellular vesicles (EV), a type of lipid vesicle, are present in the epididymis and seminal fluid. They transport some of the proteins and small RNA secreted by the epididymis or prostate to the sperm, which is critical for fertilization [9][10]. The lipid composition of EV differs in different epididymal regions [11][12]. When sperm enter the female reproductive tract, the lipid composition changes again. In vitro studies revealed that cervical mucus may select different sperm subpopulations based on lipid levels or directly affect the lipid composition of sperm when migrating through the female reproductive tract [13]. Phosphatidylcholine (PC) levels increased when sperm were exposed to female reproductive tract secretions in vivo [14]. As the sperm pass through the utero-tubal junction, they attach and aggregate along the epithelium of the isthmus until ovulation. Spermatozoa are then released from the epithelium and continue to migrate towards the oocyte [15]. Lipids are involved in the interaction of sperm with the oviduct, e.g., anandamide inhibits sperm binding and induces the release of sperm from the oviductal epithelium [16]. At the appropriate time and place sperm cells undergo capacitation and trigger the acrosome reaction (AR), eventually penetrating the zona pellucida (ZP) to fuse with the egg cell [15][17]. During these processes, the plasma membrane lipids undergo another series of alterations [18][19][20]. These, for instance, include the disruption of lipid composition and transmembrane phospholipid asymmetry, lateral diffusion of phospholipids, loss of cholesterol and reorganization of detergent-resistant structural domains observed during the capacitation process [18][21][22]. Subsequent to AR and ZP penetration, the equatorial region of the sperm cell head plasma membrane is involved in the fusion process with the oolemma, the oocyte plasma membrane (reviewed in [1]).
The functions of lipids in male fertility have received increasing attention, with many excellent studies focusing on this subject. In this review, we highlight the important role of lipid classes in sperm during fertilization. In addition, the relationship between plasma membrane lipids and male fertility is discussed, and potential lipid markers of male subfertility and infertility are emphasized. Lipids in sperm are primarily discussed, but minor contents of lipids in seminal plasma and testis are also included.
The lipid composition of the sperm plasma membrane has been elucidated for several mammalian species such as sheep [23][24], cattle [25][26][27], pig [2][14][28], mouse [29][30], rabbit [31], rat [32], horse [33], human [28], ruminantia (cattle, roe deer and Klipspringer) and feloideae species (domestic cat, Siberian tiger and fosa) [34]. Although there is considerable diversity among different mammalian species, in general, the plasma membrane contains about 70% phospholipids, 25% neutral lipids and 5% glycolipids (on molar base) [35]. In contrast to other cell types, the lipid composition of spermatozoa shows some specific characteristics. Spermatozoa have a higher proportion of neutral lipids, especially large amounts of diacylglycerol (DAG) [26][33]. Phospholipids are characterized by a large proportion of alkenyl phospholipids (plasmalogens) in choline- and ethanolamine-containing glycerol phosphates, and high proportions of highly unsaturated fatty acids such as arachidonic (20:4), docosapentaenoic (22:5) and docosahexaenoic acid (22:6) [2][36][37]. Recently, the lipid component (O-acyl)-u-hydroxy-fatty acids (OAHFA) has been identified for the first time in spermatozoa, with a carbon chain length up to 52. It was localized to the head of sperm instead of the tail region [33].
Of the lipid types present in spermatozoa, phospholipids account for the largest proportion. Phospholipids can be classified as phosphoglycerolipid (also known as glycerophospholipids (GPLs)) and sphingomyelin (SM), depending on whether the backbone is glycerol or sphingosine ( Figure 1 ). Phosphoglycerolipid can be further categorized into different subclasses depending on the different molecules attached to the glycerol backbone. The sn-1 or sn-2 position of the glycerol backbone is connected to the aliphatic acyl-, alkyl- or alkane/alkenyl- chain; the sn-3 position is combined with phosphoric acid and its derivatives [1][38]. The common types of phosphoglycerolipids in most mammals are phosphatidylcholine, phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylserine (PS), depending on the attached polar molecule [14][33][39]. Fatty acids (FAs) esterified on phospholipids can be divided into two categories based on the presence or absence of double bonds in the chain structure: (1) saturated fatty acids without double bonds (SFAs), or (2) monounsaturated fatty acids (MUFAs) with a single double bond and polyunsaturated fatty acids (PUFAs) with two or more double bonds [40].
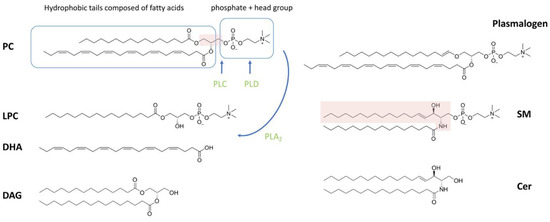
Cholesterol and diacylglycerol are the main neutral lipids in the sperm plasma membrane. Neutral lipid composition varies between species, individual males and between ejaculates [35]. Cholesterol concentrations in the seminal plasma of many species, such as rams, boars, stallions, human and domestic cats, have been investigated [37][41][42][43]. The synthesis and metabolism pathways of DAG in cells are summarized in [44].
Glycolipids are an important class of lipids present in sperm membranes, accounting for about 5-8% of total polar lipids in mammalian species [37]. Glycosphingolipids are formed by the addition of glycosidic head groups to ceramides. A more specific one is the glycolipid seminolipid, which contains sulfogalactosylglycerol in its molecular structure. Sulfogalactosylglycerolipid (SGG), also known as seminolipid, is the major anionic glycolipid found exclusively in the plasma membrane of mammalian spermatozoa [45].
The composition and structure of the sperm plasma membrane are essential in the process of spermatogenesis and sperm maturation [46][47]. During sperm maturation in the epididymis, cholesterol sulfate may act as a membrane stabilizer and enzyme inhibitor [48]. In semen, the stability of sperm membranes relies on the binding of choline phospholipids to seminal plasma proteins, which prevent the movement of phospholipids [49][50][51]. During the migration through the female reproductive tract, sperm undergo a series of biochemical and ultrastructural changes, including changes in the lipid composition of the plasma membrane (reviewed in [52]). For instance, loss of asymmetric transbilayer distribution and the substantial loss of cholesterol and phospholipid occur during capacitation and acrosome reaction (AR) [53][54][55]. The effluxed cholesterol can be bound by albumin and high-density lipoprotein in the uterine and follicular fluid [53][56]. The decrease in phospholipids is mainly due to the breakage of their hydrophobic tails and thus degradation to lysophospholipids and free FAs (22:4 n-9, 22:5 n-6) [19]. Lysophospholipids as signaling phospholipids affect male fertility [38]. Free fatty acids can increase sperm motility, viability and promote AR [57]. During capacitation and AR, sphingosine is hydrolyzed to ceramides, with the main changes occurring in species with very-long-chain polyolefin fatty acids [19]. Loss of cholesterol and phospholipids during capacitation is thought to be associated with phosphorylation of proteins occurring in the tail region of sperm cells, while lipid metabolites produced in AR accumulate in the sperm head and are thought to be involved in later fertilization processes [19][58].
Sperm–egg union is one of the most important events in sexually reproducing organisms. Lipids, as major components of the membrane, maintain sperm integrity, control membrane fluidity, provide functional cell membrane microstructural domains and provide signaling molecules. Lipids are extensively involved in a wide range of processes, from spermatogenesis and maturation, to meeting and binding to the egg cell. In this review, we summarized the effects of different lipid types described so far on the events during fertilization. We also list the effects of lipid deficiency on spermatogenesis, maturation and fertilization ability in a lipid metabolism-related knockout mouse model.
Each lipid class does not act in a separate manner; they rather participate together in concerted complex pathways through synthesis, metabolism or interconversion. Sperm morphology, motility and oxidative stress damage were frequently investigated in numerous studies on male fertility. Corresponding highly relevant lipids such as unsaturated fatty acids, plasmalogens and lysophospholipids were studied with high frequency. Unsaturated fatty acids and plasmalogens perform major antioxidant functions, while lysophospholipids are markers of sperm oxidation under peroxidative conditions. Phosphatidylcholine and phosphatidylethanolamine are the most important components of the sperm plasma membrane, and therefore their levels and ratios are the most readily detectable indicators of sperm fertilization ability. Diacylglycerols and sulfogalactosylglycerolipids are of interest because of their abnormally high content in sperm or their specific occurrence in germ cells. Phosphatidylinositols, sphingomyelin and its derivatives and lysophospholipids are frequently involved in fertilization as signaling molecules, which are essential for fertility.
Many challenges remain to be solved in the elucidation of lipid functions in fertilization. Some trace amounts of lipids or lipids that play a transient role during fertilization are difficult to detect accurately. In vitro experiments do not always exactly replicate the state of the membrane in the in vivo setting. The development of novel techniques and methods may provide more clues to lipid-regulated fertilization processes and the potential effects of deregulated lipid levels on male mammalian fertility.