Polymer nanoparticles can be loaded with active compounds entrapped within the core or adsorbed/linked onto the polymeric surface. The advantages of polymer nanoparticles as active principle delivery systems over other particular systems include high drug encapsulation efficiency, intracellular uptake, stability of encapsulated active substances, biocompatibility and biodegradability with tissue and cells, especially when prepared from biopolymers. The polymeric nanocarriers can be nanocapsules, composed of an oily core in which the drug is usually dissolved, dispersed, or embedded, surrounded by a polymeric shell that controls the release profile; and nanospheres, based on a continuous polymeric network in which the drug can be retained inside or adsorbed onto their surface.
1. Overview
The synthesis of light-responsive nanocarriers (LRNs) with a variety of surface functional groups and/or ligands has been intensively explored for space-temporal controlled cargo release. LRNs have been designed on demand for photodynamic-, photothermal-, chemo-, and radiotherapy, protected delivery of bioactive molecules, such as smart drug delivery systems and for theranostic duties. LRNs trigger the release of cargo by a light stimulus. The idea of modifying LRNs with different moieties and ligands search for site-specific cargo delivery imparting stealth effects and/or eliciting specific cellular interactions to improve the nanosystems’ safety and efficacy. This work reviews photoresponsive polymeric nanocarriers and photo-stimulation mechanisms, surface chemistry to link ligands and characterization of the resultant nanosystems. It summarizes the interesting biomedical applications of functionalized photo-controlled nanocarriers, highlighting the current challenges and opportunities of such high-performance photo-triggered delivery systems.
2. Polymer Nanoparticles
Particular interest has emerged in designing and synthesizing smart nanocarriers that respond to specific stimuli. Among them, LRNs offer outstanding opportunities for controlled drug delivery in the frontier of physic, chemistry, biology, and converging engineering fields
[1][2]. Such smart nanocarriers can be functionalized with different moieties and/or ligands at the outermost surface (or inner structure) to impart stealth effect and/or elicit specific cellular interactions to improve the safety and efficacy of the resultant nanosystems
[3][4].
Drug delivery systems triggered by external stimuli allow precise control over the timing, dosage, and drug release location from the physician or the patient. Many external stimuli such as light, ultrasound, electricity, and magnetic signals have been investigated
[2]. Nevertheless, light is a particularly appealing choice due to its easiness of control and manipulation and the long successful history of applying light to trigger a therapy. Along with high biocompatibility, the possibility of spatiotemporal control of therapeutic agents and convenience, LRNs enjoy other remarkable features like their release profiles can be regulated by adjusting light wavelength, power intensity, duration of exposure, and beam diameter
[5]. A broad range of stimuli-responsive nanocarriers with diverse sizes, shapes, and surface properties have been designed in this context
[1][5]. They include liposomes, polymer nanoparticles, micelles, dendrimers, and inorganic nanoparticles made of iron oxide, quantum dots, gold, or metal oxide frameworks
[6]. The carriers’ size is typically small, from a few tenths to a few hundred nanometers and the shape, composition, and surface chemistry can be tailored on-demand to achieve the desired LRNs
[7].
Polymer nanoparticles can be loaded with active compounds entrapped within the core or adsorbed/linked onto the polymeric surface
[8]. The advantages of polymer nanoparticles as active principle delivery systems over other particular systems include high drug encapsulation efficiency, intracellular uptake, stability of encapsulated active substances, biocompatibility and biodegradability with tissue and cells, especially when prepared from biopolymers
[9][10]. The polymeric nanocarriers can be nanocapsules, composed of an oily core in which the drug is usually dissolved, dispersed, or embedded, surrounded by a polymeric shell that controls the release profile; and nanospheres, based on a continuous polymeric network in which the drug can be retained inside or adsorbed onto their surface
[11][12].
The light source for LRNs can vary from ultraviolet (UV) (200–400 nm), visible (Vis) (400–700 nm), and near-infrared (NIR) (700–1000 nm)
[6]. The controlled drug release can be achieved through photo-isomerization, e.g., azobenzene and spiropyran (SP), photo-cleavage, e.g., coumarin-based groups, photo-crosslinking, and photoinduced rearrangement mechanisms, e.g., coumarin and cinnamoyl
[13]. These features, along with other materials’ physical and chemical properties, ranging from wettability, degradability, and electrostatics to permeability and mechanical resistance, have made these LRNs have a wide range of practical applications
[14][15].
Despite the multiple applications of LRNs at the biomedical level, the modification of surfaces with specific targeting ligands is often necessary to reduce toxicity and increase stability and bioavailability
[3]. Functionalization of nanoparticles (NPs) is the process of changing their surface chemistry by grafting chemical functional groups or molecules to achieve new capabilities, characteristics, properties, or functions
[4][16]. Chemical modification of the LRNs surface is a step-by-step process that requires physicochemical and functional characterization for each step. NPs have mainly been functionalized with thiols, disulfides, amines, nitriles, carboxylic acids, carbonyls, sulfhydryls, azides, hydroxyls, phosphines, and biomolecules
[17]. Surface modification can be achieved by non-covalent strategies based on weak hydrogen bond, electrostatic, ionic, van der Walls and hydrophobic interactions, absorption, entrapment, and layer-by-layer approaches, etc.
[18][19][20]. Non-covalent interactions have the advantage of being relatively simple and do not affect either the molecules’ structure or their interaction with biological targets but can be easily influenced by different variables, such as pH and ionic strength
[21]. In contrast, covalent interactions directly bind the molecule of interest to the NP’s reactive moieties. This approach involves a linkage reaction aided by a catalyst and is the choice over physical adsorption when long-term stability is required
[16].
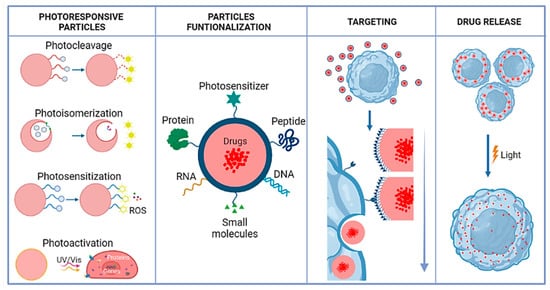
This review provides an overview of current advances in LRN drug delivery systems (Figure 1). It summarizes different light-responsive mechanisms, including photocleavage, photoisomerization, and photo cross-linking/-decross-linking. It discusses NIR for nanoscale drug delivery systems, focusing on the chemical aspects and practical examples of the functionalization process. The challenges and future perspectives of developing LRNs in controlled drug delivery applications are also assessed.
Figure 1. Schematic illustration of photosensitive polymeric nanocarriers: photosensitive mechanism, functional nanocarriers, nanocarriers targeting, and drug release.
3. Conclusions
The creative design of photocontrolled NPs has allowed control over the drug delivery process using noninvasive and spatial stimulation. Yet, its potential for translational applications and convenience is still an open question to be addressed, e.g., UV light has substantial limitations for in vitro and in vivo applications. Many biological molecules absorb these energetic wavelengths directly, preventing substantial tissue penetration and causing undesirable photochemical reactions. In addition, a challenge to achieve the controllable and reproducible fabrication of the light-activatable polymeric nanoformulations is the use of organic solvents, exotic catalysts, free radicals, and transition metal complexes that must be minimized or eliminated during material synthesis.
UV-vis and visible light to stimulate unspecific LRNs are predominant in literature. However, the use of deeper tissue penetrating NIR-light or enhancement of NPs specificity by using coating biomolecules promises to advance this technology towards the clinic. Complex macromolecular architectures and efficient coupling methods conjugated to the discovery of biologically active ligands may offer opportunities to get a vast collection of targeted systems. Their successful application against many different pathologies has demonstrated their great potential for developing personalized nanomedicines of the future.
Implementation of functionalized nanocarriers in patients requires the previous investigation of safety, biodistribution, and pharmacokinetics/pharmacodynamics in multiple animal species. However, the cost could represent a limitation for translation from the bench to the clinical. By handling nanocarriers’ surface characteristics and stealth properties, they can be transformed into smart platforms containing therapeutic and imaging agents for delivering drugs to specific cells and tissues and providing alternatives of controlled-release therapy. The continuous ongoing research on functionalized NPs envisions to improve the prevention, diagnosis and treatment of diseases. Photocontrolled functionalized systems allow unprecedented control over the delivery process using a noninvasive and spatially and temporally controllable external stimulus, holding the potential for site-specific drug delivery versatile alternatives.