Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and may progress to cirrhosis or even hepatocellular carcinoma. A number of steroid hormones are important regulators of lipid homeostasis through fine tuning the expression of genes related to lipid synthesis, export, and metabolism. Dysregulation of such pathways has been implicated in the pathogenesis of NAFLD.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) has been increasingly recognized as a worldwide health issue and is estimated to affect 20–30% of the general population
[1]. It is a progressive disease ranging from steatosis to nonalcoholic steatohepatitis (NASH), which is a risk for the development of cirrhosis and hepatocellular carcinoma (HCC)
[2][3]. Most patients with NAFLD have simple steatosis in the absence of hepatocyte injury. However, approximately 10–30% of patients with NAFLD develop NASH characterized by inflammation and fibrosis
[4]. Over the last decade, it has been shown that NAFLD is a multisystem disease, affecting several extrahepatic organs and regulatory pathways
[5]. NAFLD is tightly associated with obesity, metabolic syndrome, and type 2 diabetes
[6]. All of these complications related to NAFLD pose significant health and economic burdens on patients and society.
The pathogenesis of NAFLD is complex and described by a “multiple-hit” hypothesis, which has been proposed to supersede the outdated “two-hit” hypothesis
[7]. The “multiple-hit” hypothesis highlights the importance of genetic and epigenetic factors, nutritional factors, intestinal microbiota, insulin resistance, and hormones secreted from adipose tissue
[7][8]. All forms of NAFLD tightly correlate with hepatic and peripheral insulin resistance in epidemiological, experimental, and human studies
[9][10][11]. Insulin resistance upregulates de novo lipogenesis, inhibits β-oxidation of free fatty acids (FFAs) and increases the degradation of very-low-density lipoprotein (VLDL) in the liver, leading to an increase in hepatic fat accumulation
[12][13][14]. It also promotes lipolysis rates in adipose tissue, leading to more fatty-acid efflux to the liver
[15]. The intestinal microbiota has been found to alter bile acid metabolism to affect hepatic lipid handling and alter host immunity contributing to NASH
[16]. There is increasing evidence reporting adiponectin as an adipokine with anti-inflammatory and antifibrogenic activity protecting liver parenchyma against steatosis and apoptosis
[17].
Lipid accumulation within hepatocytes caused by these risk factors leads to steatosis, defined as the presence of fat comprising 5% of liver weight
[18]. Exaggerated lipid accumulation can cause toxicity via diverse mechanisms. For instance, toxic lipid metabolites derived from FFAs impose oxidative stress on hepatocytes
[19]. Furthermore, oxidative stress affects the function of the endoplasmic reticulum and mitochondria, as well as contributes to hepatic inflammation and fibrosis
[20].
Steroid hormones have been discovered to play important roles in lipid metabolism. Deregulation of steroid hormone level or bioactivity has been implicated in various degree of hepatocellular damages. In Cushing’s syndrome, the high circulating steroid hormone (glucocorticoid) level causes visceral obesity, insulin resistance, and hepatic steatosis
[21]. Activation of the renin–angiotensin–aldosterone system modulated by multiple steroid hormones and corresponding receptors causes liver inflammation and fibrosis
[22][23]. Moreover, steroid hormone receptor-regulated target genes are involved in both cholesterol and fatty-acid metabolism
[24] and, therefore, have been implicated in the pathogenesis of NAFLD.
2. Role of Steroid Hormones in Hepatic Steatosis and Metabolism
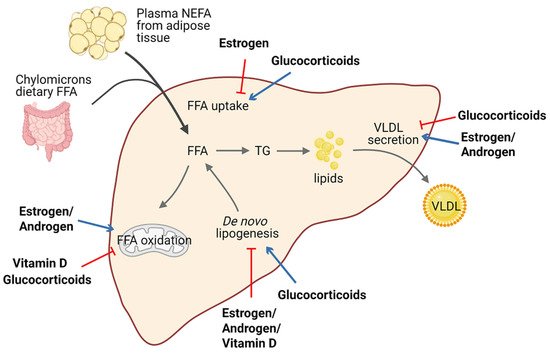
Lipid homeostasis is fine-tuned through four major pathways: uptake of circulating lipids, de novo lipogenesis, fatty-acid oxidation, and export of lipids in VLDL
[25]. Hepatic steatosis occurs when there is an imbalance between lipid acquisition and lipid disposal. Increasing data have shown that steroid hormones are involved in hepatic steatosis by modulating these processes (). We summarize steroid hormone-mediated effects of hepatic steatosis in animal models in .
It is well established that estrogen and ERs are involved in the pathogenesis of NAFLD. For example, ERα-deficient mice have disrupted lipid metabolism, such as decreased fatty-acid oxidation and increased de novo lipogenesis, leading to elevated lipid accumulation in the liver compared with normal mice
[26][27][28]. Similarly, downstream blocking of ERα contributed to higher visceral fat accumulation and reduced energy expenditure in female mice
[29][26][30]. Meanwhile, E2 treatment promoted fatty-acid oxidation in the liver by increasing the expression of the fatty-acid transport protein, carnitine palmitoyltransferase 1 (CPT-1)
[29]. In ovariectomized (OVX) female mice, estrogen replacement improves insulin sensitivity and facilitates the VLDL-mediated export of lipids from the liver by increasing hepatic VLDL-TG production
[31]. Interestingly, long-term therapy of tamoxifen, a selective estrogen receptor modulator, can increase the risk of NAFLD in breast cancer patients
[32].
A meta-analysis revealed that lower serum testosterone levels are associated with men with NAFLD
[40][41]. Testosterone deficiency in men displayed an increased accumulation of visceral adipose tissue and insulin resistance, which are factors favoring the development of hepatic steatosis
[42]. Similar results were confirmed in hepatic AR-deficient mice, indicating that AR might play a role in the suppression of NAFLD
[33][43]. Indeed, androgen/AR signaling was revealed to suppress fatty-acid synthesis by decreasing the expression of sterol regulatory element-binding protein (SREBP) and to induce insulin sensitivity by modulating phosphoinositide-3 kinase activity
[24].
Conversely, glucocorticoids drive the expression of lipogenic genes including fatty-acid synthase (
FASN) and acetyl-coA carboxylase 1 (
Acaca)
[44], stimulate de novo lipogenesis, and block VLDL secretion, thus resulting in hepatic steatosis
[45]. Corticosterone, administered to rodent models to increase basal glucocorticoids levels, also induces an increase in Cluster Determinant 36 (CD36) expression that facilitates fatty-acid uptake, favoring the progression of hepatic steatosis
[35]. Of note, patients with glucocorticoid excess may develop hepatic steatosis, as well as obesity and insulin resistance, in a significant proportion of cases
[46][47]. Moreover, fatty liver development also represents a typical side-effect of long-term systemic glucocorticoids treatment during anti-inflammatory therapy. Additionally, mice deficient in hepatic GR displayed lower hepatic TG content and elevated levels of ketone bodies in the serum, indicating that disruption of GR expression reduces steatosis in
db/
db animals partly by triggering hepatic fatty-acid oxidation and ketogenesis
[34].
Macrophage-specific deficiency of MR protects mice from hepatic steatosis and insulin resistance through the ERα/HGF/Met pathway
[36]. In addition, aldosterone deficiency attenuates high-fat feeding-induced hepatic steatosis, although the underlying mechanism remains elusive
[37]. On the other hand, elevated aldosterone level seems to enhance hepatic FFAs via modulation of lipogenesis and lipolysis
[48].
Vitamin D has been proven to protect against high-fat diet-induced fatty liver by acting on the expression of de novo lipogenesis-related genes, including
FASN and
Acaca, and fatty-acid oxidation-related genes such as the acetyl-coA oxidase (
Acox)
[38][39]. In humans, hepatic VDR expression is inversely correlated with steatosis severity
[49]. Activation of VDR in hepatic macrophages via its cognate ligand has been uncovered to ameliorate steatosis and insulin resistance in experimental studies
[50].
3f. Role of Steroid Hormones in Hepatic Inflammation and Fibrosis
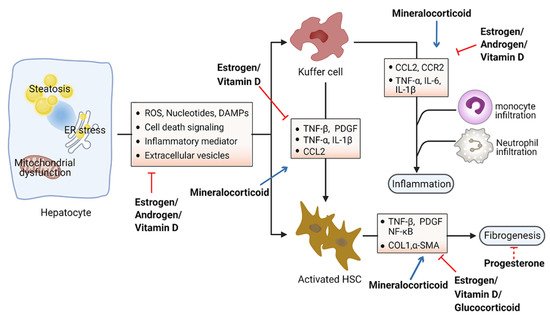
Accumulation of toxic lipids leads to ROS, endoplasmic reticulum (ER) stress, cell death, release of damage-associated molecular patterns (DAMPs), and secretion of inflammatory mediators and extracellular vesicles, which stimulate an inflammatory response in Kupffer cells and a fibrotic response in hepatic stellate cells (HSC)
[51][52]. The inflammatory process includes recruitment of macrophages and neutrophils to the site of injured tissue, followed by production of proinflammatory chemokines and cytokines such as interleukin 1 (IL-1) and tumor necrosis factor alpha (TNF-α)
[53][54]. Furthermore, initiation of the inflammatory acute-phase response of the liver is induced by these cytokines together with IL-6
[55]. Liver inflammation can lead to fibrosis that may eventually induce cirrhosis
[56]. Hepatic fibrosis is characterized by the accumulation of extracellular matrix. As a crucial driver of fibrosis, HSCs can be transdifferentiated into proliferative and fibrogenic myofibroblasts
[57]. Profibrogenic cytokines such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β) are involved in the activation of HSCs and hepatic abnormal wound repair response
[58][59][60]. Collectively, hepatocellular damage, inflammation, and fibrosis are the main characteristics of NASH
[61][62]. Numerous studies have demonstrated the important effects of steroid hormones in the pathological progression of NASH (). Here, we summarize steroid hormone-mediated effects on hepatic inflammation and fibrosis in animal models in .
Steroid hormones primarily display anti-inflammation and antifibrosis properties in the context of metabolic disorders. Estrogens have been found to directly suppress inflammatory processes in the liver. Women after menopause have decreased fatty-acid oxidation and increased lipogenesis in the liver, leading to the excessive accumulation of hepatic fat and culminating with inflammation
[63][64]. Moreover, the generation of ROS and proinflammation cytokines was prevented by estrogen signaling in hepatocytes
[65][66]. Estradiol treatment reduces hepatic inflammation in the animal model of NASH induced by methionine and a choline-deficient diet
[67]. Furthermore, female mice with estrogen deficiency induced by OVX operation were observed with enhanced levels of macrophages infiltration and proinflammatory cytokines in the liver, including TNF-α, IL-6, C–C motif chemokine ligand 2 (CCL2), and C–C motif chemokine receptor 2 (CCR2)
[68]. Hepatic fibrosis has also been shown to be associated with estrogen level. The risk of liver fibrosis increased significantly in a zebrafish experiment with ovarian senescence
[69]. Similarly, a long duration of estrogen deficiency poses a high risk of hepatic fibrosis among postmenopausal women with NAFLD
[70]. However, postmenopausal estrogen therapy may be considered for preventing the occurrence of liver fibrosis
[71]. OVX female mice fed with a high-fat and high-cholesterol diet had obvious liver fibrosis with upregulated expression of collagen I α1, which could be improved by estrogen replacement therapy
[66]. Additionally, β-LGND2, an ER-β-selective agonist, partially prevented inflammatory cell infiltration and liver fibrosis, providing a therapeutic benefit in NASH
[72].
It has been shown that testosterone may increase the level of anti-inflammatory cytokines and reduce the level of proinflammatory cytokines, thereby exerting anti-inflammatory properties in patients with diabetes mellitus or coronary artery disease
[73]. In experimental animal models of NAFLD, androgens were shown to be able to prevent NASH progression by acting on proinflammatory cytokines such as TNF-α and IL-6
[74].
Although increasing levels of progesterone have been associated with the development of systemic insulin resistance
[75], little is known regarding the role of serum progesterone in NAFLD. Early studies have shown that treatment with progesterone in rabbits exerts a protective effect on hepatocytes against vacuolization and inflammation, accompanied by a low level of fibrosis
[76].
Glucocorticoid hormones are considered the most widely used anti-inflammatory drugs. However, the effects of glucocorticoids on hepatic inflammation are still unclear, particularly in the progression of NAFLD. One study suggested that glucocorticoids may suppress the development of liver inflammation, considering that the perivascular infiltration of small mononuclear cells was diminished in the liver of mice following treatment with dexamethasone, a synthetic GR ligand
[77]. Meanwhile, glucocorticoids suppress fibrotic gene expression including collagen I α1/2 by activating GR, which differentially regulates liver injury and hepatic fibrosis in HSCs or immune cells
[77].
Aldosterone was observed to promote liver inflammation. ROS production can be stimulated by aldosterone in several tissues
[83]. Blockade of aldosterone interaction with MR by eplerenone impeded macrophage infiltration and suppressed the expression of TNF-α and multiple copies in T-cell lymphoma-1 (MCT-1) in Kupffer cells, consequently ameliorating the development of NASH in mice
[78]. Unlike the other steroids, mineralocorticoids foster the development of fibrosis by upregulating serum- and glucocorticoid-inducible kinase 1 (SGK1), which increases NF-κB, thus promoting fibrosis
[84]. Additionally, aldosterone treatment could lead to liver fibrosis in male rats independent of blood pressure via promoting the expression of several fibrosis-associated genes (e.g., TGF-β, α-SMA)
[80]. Furthermore, the expression of MR in hepatic stellate cells correlates with inflammation and fibrosis development in choline-deficient and amino-acid-defined diet-induced NASH
[79]. Specific MR blockade with eplerenone effectively ameliorated histological steatosis and hepatic fibrosis in a mouse model of NASH. These data provide the basis for therapeutic exploitation of MR blockade for treatment of NASH
[79].
Hepatic inflammation can also be affected by vitamin D through downregulation of the expression of Toll-like receptors on Kupffer cells
[85]. Vitamin D deficiency causes upregulation of inflammation and oxidative stress genes
[81]. Furthermore, restoration of vitamin D could effectively improve TNF-α-modulated immunological abnormalities in a diet-induced steatohepatitis rat model
[82]. Moreover, serum vitamin D was significantly decreased in NAFLD patients with advanced liver fibrosis, suggesting that vitamin D might be associated with the progression of liver fibrosis
[86]. Further study showed that vitamin D plays an antifibrotic role by suppressing the activation of the HSC-mediated TGF-β signaling pathway, inhibiting the accumulation of profibrotic extracellular matrix proteins
[87] and the expression of profibrotic genes including collagen and α-smooth muscle actin (α-SMA)
[88][89].