Metallic nanostructures (MNs) and metal-organic frameworks (MOFs) play a pivotal role by articulating their significance in high-performance supercapacitors along with conducting polymers (CPs). The interaction and synergistic pseudocapacitive effect of MNs with CPs have contributed to enhance the specific capacitance and cyclic stability. Among various conjugated heterocyclic CPs, polypyrrole (PPy) (prevalently knows as “synthetic metal”) is exclusively studied because of its excellent physicochemical properties, ease of preparation, flexibility in surface modifications, and unique molecular structure–property relationships. Numerous researchers attempted to improve the low electronic conductivity of MNs and MOFs, by incorporating conducting PPy and/or used decoration strategy. This was succeeded by fine-tuning this objective, which managed to get outstanding supercapacitive performances.
1. Introduction
Modern advancements in the field of flexible electronics have been actively involved in design strategies to replace conventional inorganic semiconductors by organic and hybrid inorganic materials
[1]. In this context, conjugated organic polymers, along with complementary metallic nanostructures (MNs), have been receiving much attention as promising hybrid components for flexible electronics
[2]. It was an exciting moment for the scientific community in December 2000, as the pioneering joint research works of three scientists, namely Alan J. Heeger, Alan G. MacDiarmid, and Hideki Shirakawa, collectively received the Nobel Prize in Chemistry for the discovery and the development of conductive polymers (CPs),
[3]. Due to ease of fabrication, mechanical robustness, chemical resistance, excellent electrochemical properties, and comparatively high conductivity (>10
3 S cm
−1), these CPs have crucial importance in emerging energy storage device applications as an active material. In particular, to construct advanced energy storage systems (ESSs), such as batteries and supercapacitors, functional CPs are directly incorporated as one of the energy storage active materials in addition to inorganic hybrid nanocomposites
[4][5].
Depending on the charge transfer mechanism, supercapacitors can be technically categorized into two major types, namely (i) electric double layer supercapacitors (EDLCs) and (ii) pseudocapacitors. In the former case, there is no redox process (non-Faradaic charge transfer) involved, i.e., the charges are stored progressively in the electric double layer formed at the electrode’s and electrolyte’s interface, whereas in the latter type, a series of reversible and fast sequence of redox reactions (see Equation (1), note: n = integer and e− = electron) (Faradaic charge transfer) can be noticed on the electroactive surfaces:
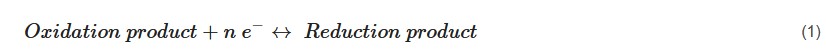
Comparatively, pseudocapacitors can accumulate greater electrochemical storage electricity and demonstrate higher energy density than EDLCs (for an illustration, see Figure 1). Accordingly, the synergistic and tunable complimenting properties of diverse nanoarchitecture metal-organic frameworks (metal oxides/phosphides/sulfides) with conjugated organic polymers, especially polypyrrole (PPy) derivatives, have found widespread application in fabricating electrochemical sensors and energy storage technologies
[6][7][8][9].
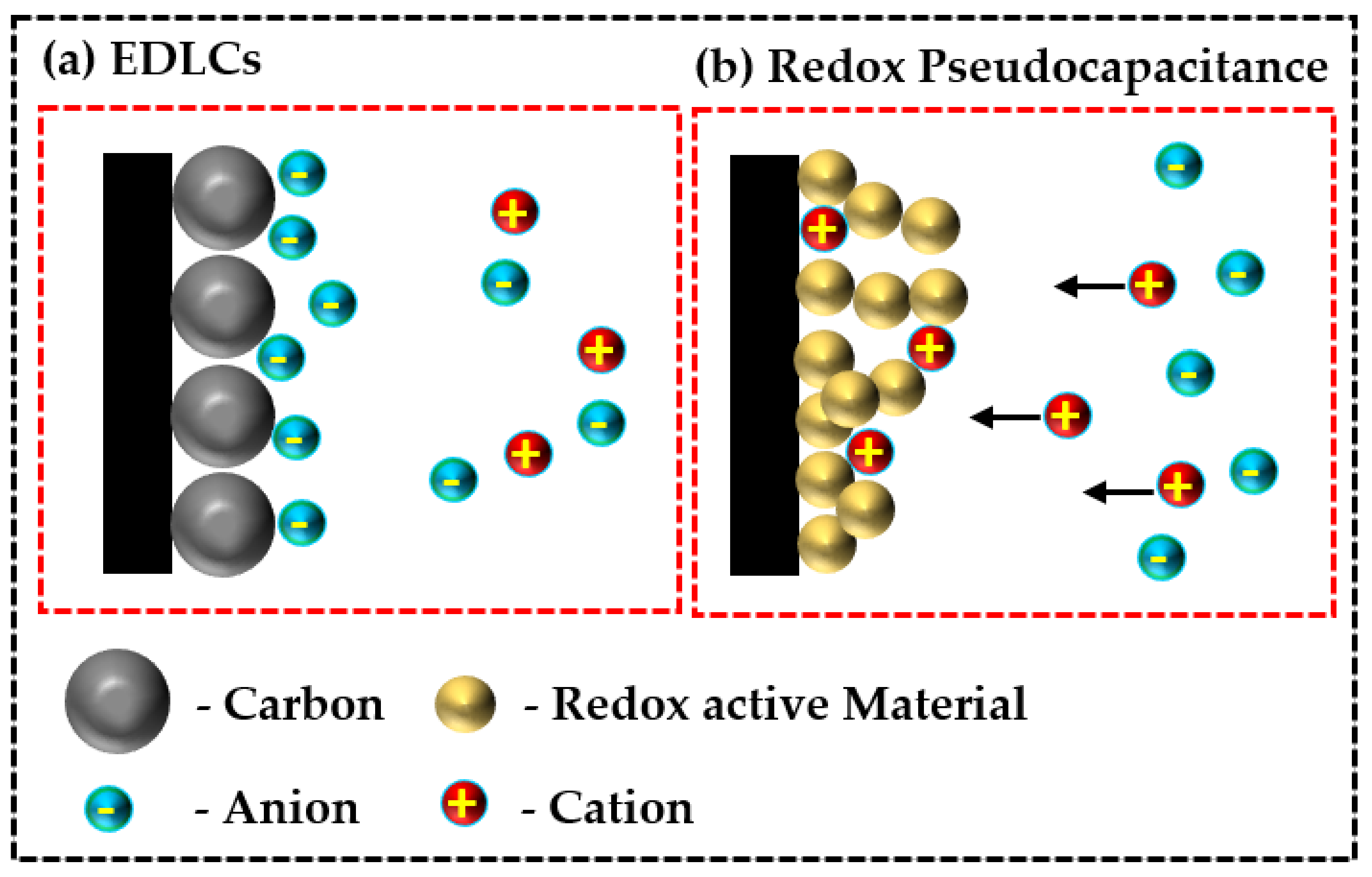 Figure 1.
Figure 1. Schematic illustration of (
a) electric double layer supercapacitors (EDLCs) and (
b) redox pseudocapacitors.
The pioneering research works on PPy from chemical, electrochemical routes by Bocchi and coworkers
[10] and Gardini
[11] was a remarkable invention, and it contributed exceptional impact to PPy chemistry. Subsequently, plentiful research works are documented on PPy derivatives. In 2006, Akar and coworkers
[12] documented optimized polymerization parameters to achieve PPy and its block copolymers with conductivities up to 4000 S cm
−1. The authors claim that the properties of these block copolymers of α,ω-diamine polydimethylsiloxane (DA·PDMS) and PPy can be regulated by adopting ratio parameters to attain unique morphology with ceric ammonium nitrate as an oxidizing agent
[12]. Pyrrole is a sensitive organic compound known for its rapid aerial oxidation to form autoxidized red tar compounds. The rapidity of aerial oxidation is even quicker when the electronic donating groups are its substituents
[13]. Despite its sensitiveness and reactivity, pyrroles can skillfully be oxidized to achieve PPy. The unique electronic properties and conductivities of PPy were enough to share the title as “organic metal/synthetic metal/metallic polymer” among other prominent conducting polymers
[14].
2. Polypyrrole-Based Hybrid Metallic Nanostructures as Electrode Materials for High Performance Supercapacitors
Hybrid metallic nanostructures embedded with PPy can exert noteworthy effects on the physico-chemical properties and the electrochemical properties due to the unique characteristics of both PPy and metallic nanostructures
[15][16]. Since the booming progress in fabricating supercapacitor electrode materials from carbonaceous network structures has been proposed and studied by various researchers to accomplish the performances beyond the limitation of carbonaceous materials
[17][18][19], Feng et al.
[20] proposed nitrogen-doped porous carbon matrix complexed with PPy. The uniformly grown PPy nanospheres on porous carbon matrix surface showed remarkably specific capacitance reaching a value of 237.5 F g
−1 with 88.53% discharge after 1000 cycles. The complimenting characteristics of notable mechanical flexibility and high capacitance of PPy was utilized to develop wearable supercapacitors by growing nanotubular arrays with carbon nano-onions on fabric material
[21]. These PPy-based hybrid nanostructures grown on fabric materials exhibited stretchable characteristics with superior energy storage capacitance (specific capacitance of 64.0 F g
−1). In addition, 99.0% capacitance was retained even at a strain of 50.0% after 500 stretching cycles
[21].
Our main aim is to focus and provide a comprehensive inventory on PPy-based hybrid metallic nanostructures as supercapacitor electrodes; a systematic survey was carried out, and comparative metal-based PPy nanocomposite electrode parameters concerning supercapacitor applications are documented. By using electrospinning technique, Li et al.
[22] successfully fabricated the hollow V
2O
5 fibers by the emulsion of vanadyl acetylacetonate, polyvinylpyrrolidone and polystyrene in N, N-dimethyl formamide followed by sintering in air at 430 °C for 30 min. Furthermore, in order to achieve hollow, capsular PPy fibers on V
2O
5, two-step vapor-phase polymerization technique was adopted and the electrodes showed appreciable specific capacitance of 203.0 mV s
−1 with over 90.0% capacitance retention after 11,000 cycles at 10.0 A g
−1 [22]. Dubal et al.
[23] reported an inexpensive and straightforward electrodeposition protocol to synthesize nano-brick structures of PPy on stainless steel (SS) substrate. The deposition of PPy nano-bricks was achieved potentiostatically at +0.9 V/SCE for 2 min. These 3D nano-brick PPy structures showed appreciable electrochemical reversibility and a large specific capacitance of 476.0 F g
−1 [23]. Shinde et al.
[24] put forward a new cost-effective chemical bath deposition (CBD) method to synthesize PPy thin film on SS substrate. These instantly grown additive-free and binder-less PPy thin films showed maximum achieved specific capacitance value 329.0 F g
−1 at 5.0 mV s
−1. Furthermore, the low equivalent series resistance (R
s = 1.08 Ω) value reflects negligible ohmic potential drop during the discharge process
[24]. Since smartly tailored SS mesh shows superior stretchability, Huang et al.
[25] fabricated PPy-based solid-state supercapacitors by electrochemically polymerizing pyrrole monomer. The fabricated supercapacitors showed an initial capacitance of 170.0 F g
−1 at a relaxed state and 214.0 F g
−1 at a 20% strain (at a specific current of 0.5 A g
−1)
[25].
The fabrication of silver nanoparticles/nanoclusters-decorated hybrid PPy (Ag@PPy) nanocomposites were done by Gan et al.
[26]. The hybrid Ag@PPy nanocomposites demonstrated an enhanced specific capacitance of 414 F g
−1 compared to that of the pure PPy electrode (273 F g
−1). Fine-sized (2–4 nm) silver nanoparticles were initially distributed homogeneously on PPy, which effectually improved the electron hopping system PPy, thus enhancing the capacitance of the PPy. Medium-sized silver nanoparticles (55–100 nm) adhered to the PPy surface, acting as a spacer that minimizes the restacking of PPy. Furthermore, the transport pathway for electrons was shortened by this unique morphology, leading to improved cycling stability and specific capacitance of hybrid Ag@PPy nanocomposites
[26]. Iqbal et al.
[27] performed the oxidative chemical polymerization of pyrrole monomer in FeCl
3 as an oxidant. The authors also prepared the binary (Co
3O
4@PPy) and ternary (Ag/Co
3O
4@PPy) nanocomposites, in situ synthesis of Co
3O
4 nanograins and silver nanoparticles along with PPy. The authors revealed spherical, tubular and globular appearances of PPy with Co
3O
4 and silver nanoparticles (some nanoparticles were also embedded inside the PPy structures). The authors showed that the ternary nanocomposites (Ag/Co
3O
4@PPy) demonstrated highest specific capacitance of 355.64 C g
−1 compared to binary (Co
3O
4@PPy) nanocomposite (280.68 C g
−1) and pure PPy (143.28 C g
−1)
[27].

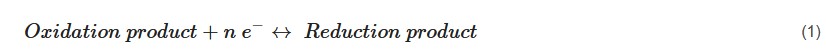 Comparatively, pseudocapacitors can accumulate greater electrochemical storage electricity and demonstrate higher energy density than EDLCs (for an illustration, see Figure 1). Accordingly, the synergistic and tunable complimenting properties of diverse nanoarchitecture metal-organic frameworks (metal oxides/phosphides/sulfides) with conjugated organic polymers, especially polypyrrole (PPy) derivatives, have found widespread application in fabricating electrochemical sensors and energy storage technologies [6][7][8][9].
Comparatively, pseudocapacitors can accumulate greater electrochemical storage electricity and demonstrate higher energy density than EDLCs (for an illustration, see Figure 1). Accordingly, the synergistic and tunable complimenting properties of diverse nanoarchitecture metal-organic frameworks (metal oxides/phosphides/sulfides) with conjugated organic polymers, especially polypyrrole (PPy) derivatives, have found widespread application in fabricating electrochemical sensors and energy storage technologies [6][7][8][9]. Figure 1. Schematic illustration of (a) electric double layer supercapacitors (EDLCs) and (b) redox pseudocapacitors.
Figure 1. Schematic illustration of (a) electric double layer supercapacitors (EDLCs) and (b) redox pseudocapacitors.