Brain-specific angiogenesis inhibitor 1 (BAI1/ADGRB1) is an adhesion G protein-coupled receptor that has been found to play key roles in phagocytosis, inflammation, synaptogenesis, the inhibition of angiogenesis, and myoblast fusion.
1. Structure of BAI1
Though BAI1 mRNA was originally reported only in the brain, it is now known that BAI1 expression is seen in a variety of tissue types in the body. Studies have shown its expression in the brain, pancreas, colon, stomach, kidney, and lung
[1][2][3][4][5][6][7][8]. Decreased expression of BAI1 in these tissues has been correlated to increased tumor growth and vascularization. Though the exact relationship and mechanism between BAI1 and angiogenesis is unknown, one study showed that overexpression of BAI1 in vascular endothelial cells resulted in increased apoptosis
[9], and the BAI-mediated inhibition of angiogenesis has been shown to occur following the interaction of its TSR domain with either CD36 scavenger receptors
[10] or α
vβ
5 integrin receptors
[11] on endothelial cells. BAI1 is a transmembrane protein and a member of the adhesion G protein-coupled receptor (GPCR) family
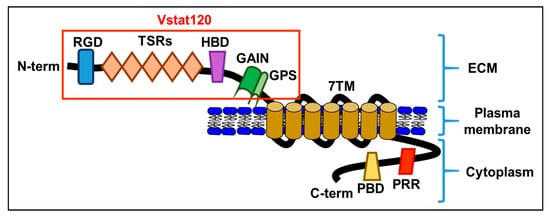
[12]. Like all adhesion GPCRs, BAI1 contains a large extracellular domain consisting of adhesive folds that allow for cell-to-matrix interactions, a GPCR autoproteolysis-inducing domain (also known as a GAIN domain), seven transmembrane regions, and an intracellular domain
[13] (
Figure 1).
Figure 1. A schematic diagram of the brain-specific angiogenesis inhibitor (BAI) domains: RGD, integrin-binding Arg-Gly-Asp motif; TSRs, thrombospondin type 1 repeats; HBD, putative hormone-binding domain; GAIN, GPCR autoproteolysis-inducing domain; GPS, GPCR autoproteolysis site; 7TM, seven-transmembrane domain; PRR, proline-rich region; PBD, PDZ-binding domain. Vstat120, secreted extracellular BAI1.
Adhesion GPCRs are found on cell surfaces as non-covalently linked heterodimers. Upon proteolysis of the GAIN domain at its GPCR proteolysis site (GPS)
[14], it creates an extracellular N-terminal fragment (NTF) and a membrane-spanning C-terminal fragment (CTF) which later re-associate in the cell membrane as heterodimers in the split GAIN domains
[15][16][17]. It has been hypothesized that the reason adhesion GPCRs split into two fragments, only to re-integrate later, is partly to create two fragments that can behave independently from one another, and partly to create adhesion-GPCR heterodimers upon re-association
[13][18].
The NTF of BAI1, also known as Vasculostatin (Vstat120)
[19], comprises the original extracellular domain of the adhesion GPCR and a portion of the GAIN domain, while the CTF comprises the original intracellular domain of the protein, the transmembrane domain, and the other portion of the GAIN domain
[13]. The NTF is a highly modular protein and plays a role at the interface of cell-to-cell and cell-to-matrix interactions
[13][20]. The CTF interacts with G proteins and other protein partners and functions in intracellular signaling
[13]. In addition to the basic features of adhesion GPCRs, BAI1 also contains a hormone-binding domain (HBD), five thrombospondin type 1 repeat (TSR) domains
[11], and an integrin-binding Arg-Gly-Asp (RGD) motif on its NTF, as well as a Gln-Thr-Glu-Val (QTEV) motif on its CTF. These additional domains allow it to interact with other proteins involved in the modification of cytoskeletal architecture, as well as interact with the localization and trafficking of other proteins
[21]. Interestingly, the CTF of BAI1 is rich in proline, which has been known to be involved with the regulation of signal transduction
[22].
2. Functions of BAI1
2.1. Anti-Tumor and Anti-Angiogenic Activity
Angiogenesis is the process of new blood vessel formation and a known hallmark of cancer, providing a conduit for transporting nutrition and oxygen to cancer cells
[23]. Since it is considered a pre-requisite for the growth of solid tumors, the inhibition of the pathways essential to driving angiogenesis has been harnessed for anti-cancer therapy
[24]. The expression of BAI1 has been found to inversely correlate with tumor neovascularization and peritumoral brain edema
[25]. This was initially identified during a screen for genes that are regulated by the tumor suppressor protein p53, and was implicated in the inhibition of neovascularization in glioblastoma
[26]. However, while the BAI1 promotor regulatory sequence contains a p53 consensus binding site
[27], the correlation between these two genes has not been substantiated in other studies
[2][28]. Recently, Zhu et al. discovered that BAI1 prevents Mdm2-mediated p53 polyubiquitination. In this study, a loss of BAI1 expression through promoter methylation resulted in reduced p53 levels and increased tumor growth in medulloblastoma, a highly aggressive pediatric brain tumor
[29]. It is important to note that epigenetic silencing due to the increased promoter methylation of BAI1 by the methyl-CpG-binding domain 2 (MBD2) has also been indicated in tumors; therefore, therapeutic strategies to silence MBD2 might lead to its reactivation
[30]. As shown in
Table 1, in addition to medulloblastoma and glioblastoma
[2], BAI1 expression plays a significant role in other cancer types, including astrocytoma, renal cell carcinoma
[28], pulmonary adenocarcinoma
[1], colorectal cancer
[6][7], bladder transitional cell carcinoma
[27], metastatic brain cancer
[31], and breast cancer
[32]. BAI1 is frequently downregulated in these different cancer types and its level of expression has often been found to be inversely correlated with tumor malignancy. BAI1 expression is commonly seen in normal brain, colon, stomach, lung, pancreas, and kidney tissues and has higher expression in normal tissues compared to its malignant counterparts. Kudo, S et al. demonstrated the effects of BAI1 expression in mice inoculated with wild-type RCCs against mice inoculated with BAI1-expressing RCCs
[4]. Overall, BAI1-expressing tumors showed significantly slower growth, decreased neovascular formation and decreased VEGF expression compared to wild-type tumors. Similarly, Duda, D.G. et al. examined the effects of induced BAI1 expression in pancreatic adenocarcinoma cells
[3]. They illustrate the fact that BAI1-expressing tumor cells had lower expression of VEGF and MMP-1, which is known to be a positive regulator of tumor angiogenesis. Furthermore, they found that induced BAI1 expression in pancreatic adenocarcinoma cells exhibited slower tumor growth in vivo and also failed to establish a stable vascular network within the tumor compared to wild-type tumor cells. Liu et al. showed that decreased expression of BAI1 is correlated with poor prognosis in lung cancer, while overexpression of BAI1 inhibited tumor growth by inducing metabolic reprogramming via the SCD1-HMGCR module
[33]. Collectively, these studies show that the reconstitution of BAI1 expression in tumors has the potential to be used as a therapeutic to reduce tumor growth and angiogenesis
[19][34].
2.2. Engulfment
In addition to angiogenesis, BAI1 plays an important role in the engulfment of apoptotic cells and infectious organisms by microglia
[35]. Its presence on microglial cells acts as a receptor for phosphatidylserine
[36], a membrane lipid that serves as an “eat me” signal on cells that should be engulfed by microglia
[37]. BAI1 is integral to the formation of and ingestion by phagosomes during phagocytosis, and BAI1 knockdown microglia show a dramatic reduction in the engulfment of both apoptotic cells and bacteria
[35]. While the TSP domains of BAI1 promote phagocytosis by binding to phosphatidylserine on the target cell, the C-terminus of BAI1 forms a trimeric complex with the proteins ELMO and Dock180
[38]. Specifically, the Dock180/ELMO complex acts as a guanine nucleotide exchange factor, resulting in the activation of the Rac GTPase protein
[39], promoting a remodeling of the microglial actin cytoskeleton and ultimately enabling the engulfment of the target
[38].
2.3. Myoblast Fusion
BAI1 also plays a role in myoblast fusion. A study by Hochreiter-Hufford et al. showed that the expression of BAI1 increased during myoblast fusion, and the overexpression of BAI1 enhanced the myoblast fusion via ELMO/Dock180/Rac1 signaling
[40]. Interestingly, this study showed that BAI1 null mice were smaller in size and had significantly impaired muscle regeneration. However, ELMO and Rac were also shown to be critical for myoblast fusion, even when BAI1 was overexpressed, as knocking down ELMO2 protein expression in C2C12 myoblasts resulted in fewer and smaller myoblasts, and the inhibition of Rac via EHT 1864 also inhibited the myoblast fusion
[40].
2.4. Synaptogenesis
BAI1 is also known to play a critical role in cell adhesion and signal transduction in the brain
[41][42]. It has been shown to regulate cytoskeletal activity and signal transduction during neuronal growth
[22] and regulate the release of neurotransmitters
[41][42]. It also plays an important role in neural development, synapse formation, and signal transduction at neuronal synapses
[43]. For example, BAI1 has been shown to regulate synaptogenesis by recruiting the Par3/Tiam1 polarity complex to synaptic sites, resulting in the activation of Rac1 GTPase, and ultimately regulating the development and plasticity of neuronal synapses and dendritic spines through the modulation of actin dynamics
[44]. However, the mechanism by which BAI1 promotes synaptogenesis is completely distinct from those mechanisms by which it inhibits angiogenesis and promotes phagocytosis
[44]. In the study by Zhu et al., they used BAI1 knockout (BAI1
−/−) mice to show that BAI1 interacts with E3 ubiquitin ligase MDM2 and prevents the polyubiquitination and degradation of the postsynaptic density (PSD) component PSD-95, which regulates synaptic plasticity. Furthermore, they showed that BAI1
−/− mice have severe defects in their hippocampus-dependent spatial learning and memory and that the restoration of PSD-95 expression in hippocampal neurons in BAI1
−/− mice rescued defects in synaptic plasticity. Overall, this suggests a potential therapeutic application of BAI1 for neurological disorders
[29].
3. Treatment Applications of BAI1
Increasing BAI1 expression has been considered a possible method of cancer therapy, and has been specifically interrogated for the treatment of glioblastoma (GBM)
[30], one of the most common and aggressive malignant brain tumors in adults
[45]. Alteration in DNA methylation is known to increase tumor malignancy
[46] and the DNA hypermethylation phenotype has been associated with GBM-harboring mutant IDH. Interestingly, BAI1 expression in GBM tissues has been shown to be regulated by methylation-mediated epigenetic silencing
[30]. Following DNA methylation, the actual silencing of gene expression is accomplished by methyl-CpG-binding proteins, which bind to methylated DNA via the methyl-CpG-binding domain (MBD), enabling the transcriptional repression domain (TRD) to silence gene expression
[47]. In a feedback loop, methylation of BAI1 in GBM results in the upregulation of MBD2 and its subsequent binding to BAI1′s methylated promoter, silencing BAI1 expression
[30].
The direct administration of BAI1 is another approach that has been utilized successfully in the preclinical space. As an example, Xiao et al. found that mice bearing GBM tumors and then treated with a recombinant adenovirus carrying the human BAI1 cDNA exhibited significantly increased survival times relative to control mice
[48]. In another study, Kang et al. demonstrated the efficient transduction and successful therapeutic application of an adenoviral vector encoding BAI1 into established human GBM xenografts in SCID mice
[45]. While the bulk of studies related to BAI1 are focused on its therapeutic effect against GBM, BAI1 has also been shown to have a therapeutic effect in other cancer types
[3][4].