1000/1000
Hot
Most Recent

Small heat-shock proteins (sHSPs) are ATP-independent molecular chaperones that interact with partially unfolded proteins, preventing their aberrant aggregation, thereby exhibiting a chaperone-like activity. Dynamics of the quaternary structure plays an important role in the chaperone-like activity of sHSPs. However, relationship between the dynamic structure of sHSPs and their chaperone-like activity remains insufficiently characterized. Many factors (temperature, ions, a target protein, crowding etc.) affect the structure and activity of sHSPs. The least studied is an effect of crowding on sHSPs activity. In this work the chaperone-like activity of HSPB5 was quantitatively characterized by dynamic light scattering using two test systems, namely test systems based on heat-induced aggregation of muscle glycogen phosphorylase b (Phb) at 48 °C and dithiothreitol-induced aggregation of α-lactalbumin at 37 °C. Analytical ultracentrifugation was used to control the oligomeric state of HSPB5 and target proteins. The possible anti-aggregation functioning of suboligomeric forms of HSPB5 is discussed. The effect of crowding on HSPB5 anti-aggregation activity was characterized using Phb as a target protein. The duration of the nucleation stage was shown to decrease with simultaneous increase in the relative rate of aggregation of Phb in the presence of HSPB5 under crowded conditions. Crowding may subtly modulate sHSPs activity.
HSPB5 (αB-crystallin) belongs to a superfamily of small heat shock proteins (sHSPs), which are ubiquitously expressed and play an important role in maintaining cellular proteostasis [1]. sHSPs bind non-native and misfolded proteins, keeping them from further aggregation and protecting the cell from toxic aggregates [1][2][3][4][5]. In addition to the exhibition of the anti-aggregation (chaperone-like) activity, these proteins are involved in many important processes in the cell, such as apoptosis, stabilization of cytoskeleton [6], regulation of muscle contraction, regulation of redox state [7], signal transduction, etc. [4][8][9][10]. Given these significant biological roles, the dysregulation of sHSPs is associated with cancer [11], cataract formation [12][13][14], and neurodegenerative diseases [5][10]. It is known that mutations in sHSPs have been directly linked to different myopathies including Charcot-Marie-Tooth disease and neuropathy [10][14][15]. Therefore, the processes of regulation of the functioning of sHSPs are very important.
Among the ten human sHSPs, HSPB5 is one of the principle members. HSPB5 is widespread in all tissues, but its concentration in the eye lens is especially high (400 mg/mL), where it interacts with HSPB4 (αA-crystallin) and forms a native hetero-oligomeric complex, α-crystallin [16]. These proteins ensure the transparency of the eye lens [17], preventing the aggregation of other crystallins and thereby protecting the lens from the development of cataracts [12]. All sHSPs, such as HSPB5, in their structure have a central α-crystalline domain (ACD) with an ordered structure, which is flanked by two variable terminal regions, C- and N- terminal domains, with a partially disordered structure, intrinsically disordered regions (IDRs) [18]. The ACD domain plays an important role in the formation of a dimer, which is considered as the building block that assembles via terminal interactions into a polydisperse ensemble under physiological conditions [9][19][20]. N-terminal regions are involved in the formation of large oligomers [18][21][22]. Carver and coworkers proposed, that the mobile C- and N-terminal regions of HSPB5 are involved in regulation of interaction with substrates; they protect ACD domain from amyloid fibril formation [20] and the flexibility of these regions, especially C-terminal regions, provides solubility for sHSPs [18][20]. The importance of the IDRs regions in sHSPs for formation of dynamic macromolecular assemblies and regulation of protein functionality has been discussed in the work [10].
HSPB5 tends to form large polydisperse assemblies ranging from 10-mer to 40-mer and higher, having very dynamic structures [19][23][24][25]. All oligomeric forms possess chaperone-like activity and easily exchange their subunits [20][26][27]. It is well accepted that a flexible dynamic quaternary structure is necessary for sHSPs activity [1][4][10][28]. Changes in the cellular environment, such as temperature [9][10][27][29], pH [30], the presence of ions [31][32], post-translational modification (phosphorylation) [33][34][35], redox environment [7][30] and crowding [19][25][32][36][37][38] also regulate chaperone activity by affecting the structural and oligomerization dynamics of sHSPs [4][5]. It has been reported that sHSP dynamics is a very complex process that includes five levels of regulation: (1) flexible domains flanking the ACD, (2) polydisperse self-oligomerization, (3) hetero- oligomerization with other sHSPs, (4) subunit exchange, and (5) regulation by the cellular environment [5][39]. As such, multiple oligomeric forms are likely relevant to function, and regulation thereof. The importance of quaternary dynamics for realization of chaperone activity of sHsps was discussed in [5][9][10][19][28][29]. However, the relationship between the dynamic variable structure and functions of sHSPs is far from being clear. The least studied is the effect of crowding on this relationship.
The cellular interior contains large concentrations of macromolecules, reaching up to 400 g/L, including proteins, nucleic acids, lipids, glycans, and solvated ions [40][41]. This means that about 40% of cell volume may be occupied by macromolecules and become physically unavailable for other molecules. A nonspecific influence of such unavailable volume on the specific biochemical reactions [42][43] was termed macromolecular crowding or excluded volume effect [44]. Theory predicts that crowding affects both the kinetics and thermodynamics of interactions between macromolecules, including protein aggregation [38][42][43][45][46][47][48].
It is usual to mimic crowded milieu in vitro by adding high concentration of suitable inert polymer or protein, so-called crowding agent, such as polyethylene glycols (PEG) of different molecular mass, polysaccharides (Ficoll, dextran), polyvinylpyrrolidone (PVP) or proteins (bovine serum albumin, lysozyme) [42]. For a long time, researchers simulated crowding in vitro by adding a single crowding agent at concentrations >100 mg/mL. Considering that the molecules of crowders are completely neutral and the effect of crowding on biochemical reactions is manifested due to steric repulsion of macromolecules, that is, the excluded volume effect (EVE). Currently, it is believed that the influence of crowding can be considered to be a mixture of entropic (excluded volume) and enthalpy based (soft interactions) effects [47][49][50][51][52]. “Soft” interactions include electrostatic, hydrophobic, and van der Waals interactions between the crowding agent and studied protein [53][54]. These interactions can be repulsive or attractive. Therefore, such soft interactions (enthalpy factor) can counteract EVE [50][55][56][57][58].
Given that in a cell crowding is created by the presence of different molecules that differ in size, shape and charge, some groups of researchers came to the conclusion that it is better to imitate physiological conditions in vitro with a mixture of several crowders (mixed crowding) [47][49][50][51][52][59]. It has been shown that two crowders can exhibit a synergistic effect, significantly enhancing the effect of each other, even when using relatively small concentrations (10–20 mg/mL) [49]. It has also been shown that small crowders create more total excluded volume in the vicinity of big crowder than in the bulk [49][58]. Sharp pointed out that when the steric effects of macromolecular crowders and small molecules like water and ions are treated on an equal footing, the effect of the macromolecules are less effective at crowding than water and ions [60]. Shah and coworkers developed a molecular thermodynamic formalism to examine the effects of size-polydispersity of crowders on aggregation reaction equilibrium. They showed that in the case of polydisperse crowders, the crowders with the largest size difference dominate the overall changes in the yield of the reaction [50].
Thus, crowding adds another level of complexity to the relationship between the activity and structural dynamics of HSPB5. Since it has a strong effect on protein–protein interactions, it should affect the conformation and self-association of the chaperone, the interaction of the chaperone with the target protein, and the aggregation of the target protein. Previously, we have shown by the analytical ultracentrifugation (AUC) method that crowding strongly affects the oligomeric state of HSPB5, HSPB6, HSPB1 and α-crystallin [25][32][36][61][62][63][64]. Thus, one might assume that crowding affects the capability of sHSPs to prevent aggregation of target proteins.
The goal of this work was to quantitatively assess the effect of crowding, including mixed crowding, on the chaperone-like activity of HSPB5. As the process of protein aggregation includes the stage of protein unfolding followed by the aggregation of unfolded protein molecules, in a general case, sHSPs can affect the unfolding stages as well as the aggregation stage. Therefore, it is very important to question what stage of the overall aggregation process (unfolding or aggregation) is rate-limiting in the selected test system.
In the present work, two test systems were selected to study the chaperone-like activity of HSPB5, one of which is based on the thermal aggregation of glycogen phosphorylase b (Phb) at 48 °C, and the other is based on dithiothreitol-induced aggregation of α-lactalbumin at 37 °C; these test systems were described earlier [65][66][67][68]. Phb exists as a dimer consisting of two identical subunits with molecular mass of 97.4 kDa each [69]. α-Lactalbumin (αLa) is a small Ca2+-binding protein containing four disulfide bridges with molar mass of 14.2 kDa. Under stress conditions, the Ca2+-depleted form of α-lactalbumin attains a classical molten globule state that aggregates amorphously [70][71][72][73]. The molten globule conformation of α-lactalbumin is thought to be a target for interacting with sHSPs [66][68][70][71][72][73][74][75][76]. The rate-limiting stages were established for the aggregation process for both proteins and the effect of HSPB5 on aggregation of these proteins was quantitatively evaluated.
We compared the effect of crowding by both individual crowders and pairs of crowders on the anti-aggregation activity of HSPB5 using Phb as a target protein. Four pairs of crowders demonstrated a synergistic effect on the activity of HSPB5. This study has provided insights into the mechanism of chaperone function under crowded conditions.
It is well known that the dynamics and polydispersity of oligomers play an important role in the functioning of α-crystallins [10][29][77][78]. It was reported that subunit exchange promotes structural reorganization within the homo-oligomers of αB-crystallin [27] (see review [29]). The equilibrium between oligomeric forms is very sensitive to many factors such as temperature [1][27][79][80][81], post-translational modifications (phosphorylation) [33][35][82], divalent cations [31][32], crowding conditions [25], presence of target protein [25], and many others. Benesh and colleagues [77] suggested that the quaternary structure of α-crystallins is modulated by the assembly of oligomers from monomers or from dimers and there is an exchange between these forms, that have different conformation and chaperone-like activity [77]. Aquilina and coworkers showed that the population of αB-crystallin oligomers from the bovine eye lens contains oligomer consisting of an even and odd number of subunits [83]. They concluded that a monomer is the main building block of this assembly. Considering the monomers as the most active species, one can explain why αB-crystallin can prevent protein aggregation at very low stoichiometric ratio compared to the target protein. Such effective stoichiometry is possible in the cell, since the unfolded target proteins are often present at low concentrations and aggregate slowly (for example, in the lens). Therefore, a high concentration of active monomer species is not required to prevent the unfolding and aggregation of the target protein [29].
In the present work the stoichiometry of the HSPB5–target protein complex (S0) have been determined for two target proteins using Equation (3). In the case of heat-induced aggregation of Phb we have shown that the dependence of the Kagg/Kagg,0 value on the ratio of molar concentrations of HSPB5 and Phb ([HSPB5]/[target protein]) is complex, and the stoichiometry of the resulting chaperone–target protein complexes is variable (Figure 1). Possibly, the obtained complexity of the above dependence is associated with a change in the quaternary structure of HSPB5 with varying molar ratio [HSPB5]/[Phb]. We suggest that the rather complicated dependence of the Kagg/Kagg,0 value on the molar ratio [HSPB5]/[Phb] (Figure 1) is associated with: (1) the change in the oligomeric state of the chaperone with an increase in its concentration, and (2) possible variations in the affinity of HSPB5 species with respect to the denatured/aggregated protein. We assume that the lower the concentration of HSPB5, the greater is the fraction of “traveling monomers” in the solution at 48 °C [27] and, therefore, the more HSPB5 oligomers consist of monomeric building blocks [77]. With increasing concentration of HSPB5, the proportion of dimers and oligomers constructed from dimeric building blocks grows. The chaperone function of these two states is different: the monomeric substructural state has greater exposed hydrophobic surface area and is consequently more active [77]. All the above mentioned is likely to underlie the obtained value of the stoichiometry of the chaperone–target protein complexes at the initial stages of Phb aggregation at 48 °C at low ratios of molar concentrations [HSPB5]/[Phb] (in the excess of the molar concentration of Phb): when one monomer of HSPB5 (with the molecular mass of 20.2 kDa) is complexed with over three Phb monomers (with the molecular mass of 97.4 kDa each).
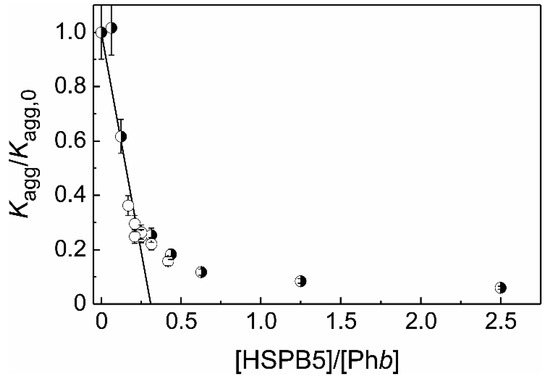
Figure 1. The suppression of Phb aggregation by HSPB5. The dependence of Kagg/Kagg,0 ratio on the ratio of molar concentrations of HSPB5 and Phb (x = [HSPB5]/[Phb]). Open circles correspond to Kagg/Kagg,0 values at constant HSPB5 concentration (0.025 mg/mL). Half-filled circles correspond to Kagg/Kagg,0 values at constant Phb concentration (0.4 mg/mL). The solid line is calculated from the equation Kagg/Kagg,0 = 1 − x/S0 at S0 = 0.31.
When studying the kinetics of DTT-induced aggregation of αLa (a protein with a molecular mass of the monomer of 14.2 kDa, smaller than that of HSPB5 subunit) in the presence of HSPB5 (Figure 2), we obtained an even lower value of the stoichiometry of the chaperone–target protein complexes, namely less than 0.011. According to the sedimentation velocity analysis we conclude that suboligomeric forms of HSPB5 (monomeric or dimeric) may interact with the target protein, DTT-denatured αLa, forming relatively small oligomers (see Table 1). However, the high-order oligomeric HSPB5–αLa complexes may be present. According to Hayashi and Carver the monomeric form of HSPB5 may be its most chaperone-species active [29]. However, the presence of monomers can increase the hydrophobicity of HSPB5 and decrease its solubility. Therefore, for the chaperone-like activity, the balance between monomeric and oligomeric forms is very important.
Table 1. Estimation of the molar mass and s20,w values of the complexes between HSPB5 and αLa denatured by 20 mM DTT (0.1 M Na-phosphate buffer, 0.01 M NaCl, pH 6.8, 37 °C).
| [HSPB5] (mg/mL) | s20,w (S) | Friction Ratio, f/f0 | Molecular Mass (kDa) |
|---|---|---|---|
| 0 | 2.9 ± 0.8 6.1 ± 0.4 |
2.7 | |
| 0.00045 | 1.85 ± 0.13 | 1.614 | 25.4 |
| 0.0015 | 2.0 ± 0.1 | 1.545 | 26 |
| 0.0075 | 1.9 ± 0.2 2.4 ± 0.3 |
1.925 | 33 47 |
| 0.03 | 1.6 ± 0.2 2.0 ± 0.1 |
1.836 | 23.9 37.4 |
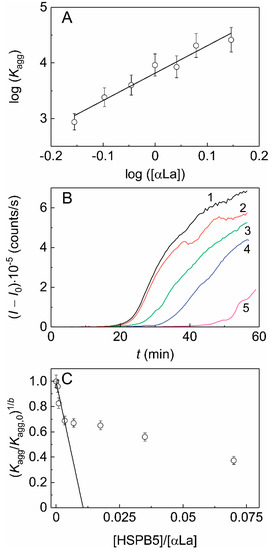
Figure 2. The effect of HSPB5 on DTT-induced aggregation of α-lactalbumin (αLa). (A) The relationship between parameter Kagg and the initial concentration of αLa in coordinates {log([αLa]); log(Kagg)}. The slope of the linear fitting is equal to parameter b (b = 4.9 ± 0.5). (B) The dependences of the light scattering intensity (I) on time for αLa aggregation ([αLa] = 1 mg/mL, 0.1 M Na-phosphate buffer, pH 6.8, 20 mM DTT, 37 °C) in the absence of HSPB5 (curve1) and in the presence of the following concentrations of HSPB5: (2) 0.001, (3) 0.0025, (4) 0.0075 and (5) 0.025 mg/mL. (C) The dependence of the relative parameter (Kagg/Kagg,0)1/b on the ratio of the molar concentrations of the chaperone and the target protein (x). The linear fitting is drawn according to Equation (3).
In the present work, to quantify the effect of crowding on the chaperone-like activity of HSPB5, we selected parameter Kagg characterizing the acceleration of the aggregation process during the nucleation stage. Phb aggregation at 48 °C was chosen as a test system. Table 2 shows the effect of crowding on the kinetic parameters of Phb thermal aggregation. When considering the effect of crowding on Phb aggregation in the presence of HSPB5, it must be emphasized that all crowders used, as well as their pairs, increase the value of parameter Kagg as compared to that in the buffer, although to a different extent. That is, the aggregation of the target protein in the presence of HSPB5 under the conditions of crowding created by all used crowders or their mixtures is accelerated compared to the aggregation process in the buffer. Thus, the data obtained by the dynamic light scattering (DLS) method suppose that crowding reduces the chaperone-like activity of HSPB5.
Table 2. Kinetic parameters for aggregation of Phb (0.4 mg/mL) in the presence of HSPB5 at a concentration of 0.025 mg/mL at 48 °C (0.03 M Hepes buffer, 0.1 M NaCl, 0.2 mM EDTA, pH 6.8).
|
Additions |
Kagg ((counts/s)/s2) |
t0 (s) |
Kagg/K0agg1 |
|
Without addition of crowding agents |
|||
|
– |
0.123 ± 0.007 |
323 ± 9 |
1.0 |
|
Action of individual crowding agents |
|||
|
PEG20kDa 25 mg/mL |
1.85 ± 0.04 |
287 ± 2 |
15.0 ± 0.9 |
|
PVP10kDa 25 mg/mL |
0.28 ± 0.01 |
266 ± 5 |
2.3 ± 0.2 |
|
PVP25kDa 25 mg/mL |
0.52 ± 0.02 |
232 ± 5 |
4.2 ± 0.3 |
|
Ficoll70kDa 75 mg/mL |
0.144 ± 0.015 |
252 ± 12 |
1.17 ± 0.14 |
|
Combined action of crowding agents |
|||
|
PEG20kDa 25 mg/mL + Ficoll70kDa 75 mg/mL |
1.37 ± 0.05 |
196 ± 4 |
11.1 ± 0.8 |
|
PVP10kDa 25 mg/mL + Ficoll70kDa 75 mg/mL |
1.69 ± 0.05 |
263 ± 3 |
13.7 ± 0.9 |
|
PVP25kDa 25 mg/mL + Ficoll70kDa 75 mg/mL |
3.76 ± 0.21 |
240 ± 3 |
30.6 ± 2.4 |
|
PVP10kDa 25 mg/mL + PEG20kDa 25 mg/mL |
4.00 ± 0.22 |
227 ± 3 |
32.5 ± 2.6 |
|
PVP25kDa 25 mg/mL + PEG20kDa 25 mg/mL |
6.76 ± 0.29 |
203 ± 2 |
55 ± 4 |
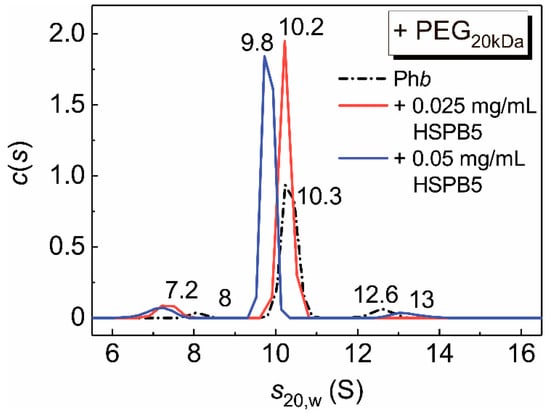
Figure 3. The c(s) distributions for the mixtures of Phb (0.5 mg/mL) and HSPB5 in the presence of PEG20kDa (25 mg/mL). The following concentrations of HSPB5 were used: 0 (black), 0.025 mg/mL (red) and 0.05 mg/mL (blue). The c(s) distributions were corrected to the standard conditions. Rotor speed was 48,000 rpm. The total time of the experiment at the elevated temperature was 90 min.
Figure 4. Interaction of HSPB5 with Phb as a target protein at 48 °C under crowded conditions arising from the presence of the mixture of PEG20kDa (25 mg/mL) and PVP25kDa (12.5 mg/mL). The differential sedimentation coefficient distributions, c(s), for Phb (0.4 mg/mL; dash dot blue line), HSPB5 (0.26 mg/mL; dash red line) and its mixture (solid black line) are presented. The c(s) distributions were obtained at 48 °C and transformed to standard s20,w distributions. Rotor speed was 48,000 rpm. The total time of the sedimentation experiment at the elevated temperature was about 90 min.
That is, under mixed crowding conditions, although a 55-fold increase in the value of parameter Kagg was registered by DLS (compared with the buffer, Table 2), complexes formed by the suboligomeric forms of HSPB5 with the target protein are retained in the solution. This fact is consistent with our earlier data on the interaction of HSPB5 with Phb at 48 °C in the presence of the pair of crowders, PEG20kDa and TMAO [25]. The presence of a target protein may stimulate the dissociation of large HSPB5 assemblies. However, the existence of high-order oligomeric HSPB5–Phb complexes cannot be ruled out. The obtained results support our previous data on the formation of the complexes between dissociated forms of bovine lens α-crystallin and an apoform of Phb [63], or Phb denatured by ultraviolet radiation [62], apart from the high order complexes. The presence of two types of complexes formed by α-crystallin and target proteins, which differ in their sensitivity to crowding and aggregation, was reported in the work [62]. High molecular mass complexes are aggregation-prone, whereas complexes formed by small suboligomeric forms of chaperone with a target protein are more resistant to aggregation under crowding conditions [36,62]. We suggested that these small complexes are responsible for the realization of the chaperone-like activity of HSPB5 or α-crystallin under crowded conditions [25,36,62]. Our results are consistent with this idea.
Thus, we showed that by changing the combination of different crowding agents, almost spherical crowders, like Ficoll, or linear polymers, like PVP, it is possible to regulate (moderate) the activity of the chaperone. Our studies show that adding even one crowder to an existing one dramatically changes the effectiveness of the crowding. Parameter Kagg increases in 13.7–55 times in the presence of those pairs of crowders that exhibit synergism (Table 2). On the one hand, this can be explained by the increase (strengthening) of the excluded volume effect, which leads to acceleration of the target protein aggregation. On the other hand, it is currently believed that, in addition to the excluded volume effect (steric repulsion of macromolecules – the entropy factor), the forces of weak interaction between protein molecules and crowder molecules (enthalpy factor) play a significant role in crowded environment [49,50,97–100]. In addition, biopolymers (proteins) capable of reversibly changing the state of association/dissociation or accepting an expanded or compact state of the quaternary structure can change the level of excluded volume in cells [101,102]. It is assumed that proteins with such properties, through their ability to directly influence the degree of excluded volume, will dynamically regulate the functions of proteins in biological media [102]. Small heat shock proteins, such as HSPB5, can take extended or compact states [9,20] and change the state of association, therefore can affect the effective level of excluded volume. Thus, crowding adds another level of complexity to the relationship between the activity and structural dynamics of sHSPs and may subtly modulate sHSPs activity.