Several studies have shown that mesenchymal stromal/stem cells (MSCs) exert their neuroprotective and neurorestorative efficacy via the secretion of neurotrophic factors. Based on these studies, many clinical trials using MSCs for the treatment of neurological disorders have been conducted, and results regarding their feasibility and efficacy have been reported.
1. Introduction
Mesenchymal stromal/stem cells (MSCs) have been the focus of new cell therapy development due to their potential to treat neurological disorders
[1]. MSCs were first discussed in 1991 when they were introduced by Caplan as mesenchymal cells in bone marrow
[2]. Now, MSCs have been isolated from several sources, including bone marrow (BM), the umbilical cord (UC), umbilical cord blood (UCB), dental pulp (DP), and adipose tissue (AD)
[1][3][4][5][6][7][8].
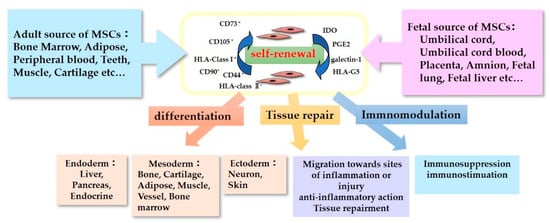
The characteristics of MSCs are defined by a set of criteria that form the basis for their clinical use (
Figure 1). The International Society for Cellular Therapy proposed the criteria for defining human MSCs
[9][10]. Firstly, the MSCs must be plastic-adherent when maintained in standard culture conditions. Secondly, they must express CD105, CD73, and CD90, but not CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR surface molecules. Thirdly, MSCs must be able to differentiate into adipocytes, chondroblasts, and osteoblasts in vitro. Immunomodulatory effects are the most important and popular property of MSCs in their clinical use
[11]. MSCs lack HLA-class II expression and do not express the co-stimulatory surface antigens CD80 or CD86, which activate T-cells
[12]. As a result, these cells are able to escape from activated T-cells. MSC-mediated immunomodulation results from the MSC secretome, which includes components such as indoleamine 2,3-dioxygenase (IDO), PGE2, galectin-1, and HLA-G5
[13]. With these anti-inflammatory properties, MSCs could be useful therapeutic candidates for use in the treatment of neurological disorders accompanying inflammation.
Figure 1. Characteristics of Mesenchymal stromal/stem cells (MSCs).
The tissue repair properties of MSCs are also important to their neurorestorative effect. The neurorestorative and neuroprotective effects of MCSs regarding tissue repair can be divided into two main mechanisms: (1) neurogenic differentiation and eternal cell replacement and (2) the secretion of neurotrophic factors
[14]. Regarding the former, we have observed in our experiments that MSCs do not engraft and differentiate into neural cells, and they disappear within two weeks of administration in non-immunocompromised mouse models
[15]. In contrast, we found that UC-MSCs that secrete neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and hepatocyte growth factor (HGF) but not nerve growth factor (NGF) attenuate brain injury
[15][16]. This MSC paracrine effect is expected to contribute toward their use in therapeutics for neurological injuries. Many studies using neurological disorder models have reported improvements in the studied conditions after the administration of MSCs, and clinical studies using MSCs to treat neurological disorders have been already conducted.
2. Clinical Application of MSCs for Neurological Disorders
Based on the mechanisms suggested by the basic experiments mentioned above, several clinical trials using MSCs for neurological disorders have been conducted, and the recent clinical reports are summarized in this review.
Most of these clinical studies were performed with adult participants, while trials focusing on cerebral palsy were performed with children. Regarding the origin of MSCs; BM, UC, UCB, and AD sources have all been used. In addition, DPSCs are used for clinical trials for neurological disorders
[17][18]. As for the administration of MSCs, most clinical studies adopt IV and/or IT. These clinical trials have mainly reported on the feasibility and efficacy of MSC therapies for neurological disorders, with some reporting adverse events, such as fever, vomiting, and headaches, while severe adverse events have not been observed.
2.1. Ischemic Stroke
The recent clinical reports using MSCs for the treatment of ischemic stroke are summarized in
Table 1. Some studies have reported on the safety and feasibility of BM-MSCs in patients with ischemic stroke injury
[19][20][21][22]. In these clinical trials, patients received intravenous injections of BM-MSCs, and an improvement in neurological functioning was observed, while no treatment-related adverse events were seen. Qiao et al. highlighted the safety and feasibility of the co-transplantation of neural stem/progenitor cells (NSPCs) and UC-MSCs in patients who had suffered from an ischemic stroke
[23]. In the study, no tumorigenesis was found during a two-year follow-up, and the neurological functions, disability levels, and daily living abilities of the patients had improved. Jiang et al. reported on the safety and efficacy of UC-MSCs delivered via a catheter to a near-lesion site for treating an infarction in the middle cerebral artery territory
[24]. UC-MSCs were infused via catheterization in the M1 segment of the middle cerebral artery. Cell delivery was performed successfully in all the patients, and no major accidents were observed. After this cellular therapy, two of the three ischemic stroke patients demonstrated improved muscle strength. These reports suggest that the transplantation of MSCs in subjects with ischemic stroke is safe and may promote neurological improvement. On the other hand, Nagpal et al. conducted a clinical trial using DPSC for stroke called TOOTH (The Open study of dental pulp stem cell Therapy in Humans) and are investigating the use of autologous stem cell therapy for stroke survivors with chronic disability
[18].
Table 1. Summary of recent clinical trials using MSCs for ischemic stroke.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Chung et al. [19] |
Ischemic stroke
(Phase 3) |
BM |
39 |
15 |
68
(28–83) |
IV |
1 × 106/kg |
1 |
Lower extremity motor functional recovery
after 3 months |
No |
| Bhasin et al. [20] |
Ischemic stroke |
BM |
6 |
6 |
42.8
(20–60) |
IV |
5–6 × 107 cells |
1 |
Improvement in the activities of daily living (ADL) after 156 and 208 weeks |
No |
| Qiao et al. [23] |
Ischemic stroke
(Phase 1/2) |
UC |
6 |
0 |
56.17
(3–85) |
IVIV + IC |
IV:MSC 0.5 × 106/kg
IC:MSC 5 × 106 cells
NSPC 6 × 106 cells at one-week interval |
4
or
1 + 3 |
Improvement in the neurological functions and ADL after 3, 12, 24 months |
Fever, dizziness |
| Jiang et al. [24] |
Ischemic and hemorrhagic stroke |
UC |
4 |
0 |
40–59 |
IA (intra-arterial) via catheterization |
2 × 107 cells |
1 |
Motor functional recovery and improvement in the ADL after 3 and 6 months |
No |
| Bhasin et al. [21] |
Ischemic stroke |
BM |
20
MSC6
MNC14 |
20 |
45.1 |
IV |
5–6 × 107 cells |
1 |
Improvement in the ADL
after 8 and 24 weeks |
No |
| Honmou et al. [22] |
Ischemic stroke
(Phase 1) |
BM |
12 |
0 |
59.2
(41–73) |
IV |
0.6–1.8 × 108 cells |
1 |
Incremental daily rate of change in the disability scales
during 12 months |
Fever, nausea, itching |
2.2. Spinal Cord Injury
In most of the clinical trials involving MSC treatment for spinal cord injury (SCI), MSCs were administered via intrathecal or direct infusion to the injured lesion (
Table 2). Vaquero et al. reported that patients administered BM-MSCs showed variable clinical improvements in sensitivity, motor power, spasms, spasticity, neuropathic pain, sexual function, and/or sphincter dysfunction, regardless of the level/degree of injury, age, or time elapsed since the SCI
[25]. Hur showed the effects and safety of the intrathecal transplantation of autologous AD-MSCs in patients with SCI. Over the 8 months of follow-up, patients who received intrathecal transplantation of autologous AD-MSCs for SCI treatment did not experience any serious adverse events, and several patients showed mild improvements in neurological function
[26]. Transplanting collagen scaffolds with human UC-MSCs has also been reported to have therapeutic potential as a treatment for SCI. Collagen scaffolds with human UC-MSCs were transplanted into the injury site directly, and the recovery of sensory and motor functions was observed in both patients
[27]. Oh et al. reported on the injection of autologous BM-MSCs into the intramedullary area and subdural space and concluded that this single MSCs application was safe, but it had a very weak therapeutic effect compared with multiple MSC injections
[28]. Therefore, further clinical trials to enhance the effect of MSCs are necessary in the future.
Table 2. Summary of recent clinical trials using MSCs for SCI.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Xiao et al. [27] |
Spinal cord injury
(Phase 1) |
UCB |
2 |
0 |
28, 30 |
Transplantation into the lesion with collagen scaffolds |
4 × 107 cells |
1 |
Motor functional recovery after 3, 6, 12 months
Sensory functional recovery after 2, 4, 12 months |
No |
| Vaquero et al. [25] |
Spinal cord injury
(Phase 2) |
BM |
11 |
0 |
44.91
(28–62) |
IT |
100 × 106 cells at 3 months interval |
3 |
Motor, sensory and bladder–bowel functional recovery
after 4, 7, 10 months |
No |
| Vaquero et al. [29] |
Post-traumatic syringomyelia
(Phase 2) |
BM |
6 |
0 |
39
(30–50) |
Direct injection into the lesion |
300 × 106 cells |
1 |
Achieving reduction of syrinx and valiable clinical improvements after 6 months |
No |
| Vaquero et al. [30] |
Spinal cord injury
(Phase 2) |
BM |
10 |
0 |
42.2
(34–59) |
IT |
30 × 106 cells at 3-months interval |
4 |
Motor, sensory and bladder–bowel functional recovery after 3, 6, 9, 12 months |
Headache,
puncture pain |
| Satti et al. [31] |
Spinal cord injury
(Phase 1) |
BM |
9 |
0 |
31.6
(24–38) |
IT |
1.2 × 106/kg at 4 weeks interval |
2 or 3 |
Only safety assessment |
No |
| Oh et al. [28] |
Spinal cord injury
(Phase 3) |
BM |
16 |
0 |
40.9
(18–65) |
Direct injection into the lesion + IT |
1.6 × 107 cells
3.2 × 107 cells |
1 |
Very weak therapeutic efficacy after 6 months |
Sensory deterioration, muscle rigidity, tingling sense |
| Hur et al. [26] |
Spinal cord injury
(Phase 1) |
AD |
14 |
0 |
41.9
(20–66) |
IT |
3 × 107 at 1-month interval |
3 |
Motor and sensory functional recovery after 8 months |
Nausea, vomit, headache |
| Mendonça et al. [32] |
Spinal cord injury
(Phase 1) |
BM |
14 |
0 |
35.7
(23–61) |
Direct injection into the lesion |
5 × 106 cells/cm3 per lesion volume |
1 |
Motor, sensory, and bladder–bowel functional recovery after 6 months |
Low-intensity pain at the incision site,
cerebrospinal fluid leak |
| Cheng et al. [33] |
Spinal cord injury
(Phase 2) |
UC |
10 |
34 |
35.3
(19–57) |
Direct injection into the lesion |
2 × 107 cells at 10 days interval |
2 |
Motor, sensory, and bladder functional recovery after 6 months
Superior efficacy than that of rehabilitation therapy |
Radiating neuralgia |
| Dai et al. [34] |
Spinal cord injury
(Phase 1/2) |
BM |
20 |
20 |
22–54 |
Direct injection into the lesion |
20 × 106 cells |
1 |
Motor, sensory, and bladder functional recovery after 6 months |
Fever, headache, pain |
| Karamouzian et al. [35] |
Spinal cord injury
(Phase 1/2) |
BM |
11 |
20 |
33.2
(23–48) |
IT |
0.7–1.2 × 106 cells |
1 |
Possible efficacy in the motor and sensory function |
No |
2.3. Cerebral Palsy
Recently, MSCs have been emerging for use in potential new therapeutic treatments for children with cerebral palsy. The recent clinical reports using MSCs for the treatment of cerebral palsy are summarized in
Table 3. Huang et al. reported on a randomized, placebo-controlled trial of UCB-MSC infusion in children with cerebral palsy
[36]. The infusion group was comprised of 27 patients, each of whom received four infusions of UCB-MSCs and basic rehabilitation treatment, whereas another 27 patients were assigned to the control group and received 0.9% normal saline and basic rehabilitation treatment. The changes in the gross motor and comprehensive functional scale in the UCB-MSC infusion group were significantly higher than those in control group at 3-, 6-, 12-, and 24-months post treatment. Liu et al. investigated whether BM-MSCs and BM-mononuclear cells (BM-MNCs) had any difference in curative effect regarding their use in the treatment of cerebral palsy. Their results indicated that BM-MSC transplantation for the treatment of cerebral palsy is safe and can improve gross and fine motor function significantly when compared with the results of BM-MNC treatment
[37]. Cerebral palsy and its associated conditions can cause significant economic burdens to families. Therefore, clinical trials that may lead to new cell therapy strategies should be further investigated.
Table 3. Summary of recent clinical trials using MSCs for cerebral palsy.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Gu et al. [38] |
Cerebral palsy
(Phase 1/2) |
UC |
19 |
20 |
4.29 |
IV |
4.5–5.5 × 107 cells
at 7-day intervals |
4 |
Gross motor and comprehensive functional recovery and improvement in the ADL after 3, 6, 12 months |
No |
| Ahn et al. [39] |
Intraventricular hemorrhage
(Phase 1) |
UCB |
9 |
0 |
11.6
(7–15)
(days) |
Intraventricular |
5 × 106/kg
or
1 × 107/kg |
1 |
Only safety assessment |
No |
| Huang et al. [36] |
Cerebral palsy
(Phase 1/2) |
UCB |
27 |
27 |
7.4
(3–12) |
IV |
5 × 107 cells at 7-day intervals |
4 |
Gross motor and comprehensive functional recovery after 3, 6, 12, 24 months |
No |
| Liu et al. [37] |
Cerebral palsy
(Phase 1/2) |
BM |
MSC 33
MNC34 |
35 |
7–132
(months) |
IT |
1 × 106/kg at 3–4-day intervals |
4 |
Motor functional recovery after 12 months |
No |
| Wang et al. [40] |
Cerebral palsy
(Phase 4) |
UC |
16 (8 twins) |
0 |
6.29
(3–12) |
IT |
1–2 × 106 cells at 3–5-day intervals |
4 |
Motor functional recovery after 1 and 6 months |
No |
| Wang X et al. [41] |
Cerebral palsy |
BM |
46 |
0 |
6–180
(months) |
IT
Intra-Parenchymal |
2 × 107 cells
4 × 107 cells
at 5-day intervals |
2 + 1
or
4 |
Gross motor functional recovery after 1, 6, 18 months |
No |
2.4. Amyotrophic Lateral Sclerosis (ALS)
ALS is a fatal neurodegenerative disease characterized by the degeneration of motor neurons in the brain and spinal cord, resulting in progressive muscle weakness and respiratory failure. The recent clinical reports using MSCs for the treatment of ALS are summarized in
Table 4. Berry et al. highlighted the safety and efficacy of neurotrophic factor (NTF)-secreting MSCs (NurOwn
®, autologous bone marrow-derived MSCs, induced to secrete NTFs) delivered by combined intrathecal and intramuscular administration to participants with ALS in a phase 2 randomized controlled trial
[42]. The rate of disease progression (Revised ALS Functional Rating Scale (ALSFRS-R) slope change) in the overall study population was similar in the treated and placebo participants, while in a prespecified rapid progressor subgroup, the rate of disease progression improved at early time points. Furthermore, CSF neurotrophic factors increased, and associated inflammatory biomarker levels decreased in the treated participants post-NTF-secreting MSC transplantation. Another report showed that intrathecal and intramuscular administration of BM-MSC secreting neurotrophic factors in patients with ALS is safe and may provide clinical benefits
[43]. Syková et al. demonstrated that the intrathecal application of BM-MSCs in ALS patients is a safe procedure and that this treatment could slow down the progression of the disease; a reduction in ALSFRS decline at three months after application was observed which, in some cases, persisted for six months
[44]. Oh et al. reported that two repeated intrathecal injections of autologous BM-MSCs was a safe and feasible treatment for ALS patients throughout the duration of a 12-month follow-up period
[45]. These results support the possibility that the use of MSCs in ALS patients could slow down the progression of the disease.
Table 4. Summary of recent clinical trials using MSCs for ALS.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Berry et al. [42] |
ALS
(Phase 2) |
BM-NTF |
36 |
12 |
51.1
(26–71) |
IM + IT |
IM: 48 × 106 cells
IT: 125 × 106 cells |
1 |
Improvement in the rate of disease
progression after 6 months |
Headache, fever, back pain, injection site bruising |
| Syková et al. [44] |
ALS
(Phase 1/2) |
BM |
26 |
0 |
51.2
(33–64) |
IT |
15 ± 4.5 × 106 cells |
1 |
Slowing down of the diseaseprogression after 3, 6, 9 months |
Headache |
| Staff et al. [46] |
ALS
(Phase 1) |
AD |
27 |
0 |
36–75 |
IT |
1 × 107, 5 × 107, 5 × 107 × 2, 1 × 108, 1 × 108 × 2 |
1 or 2 |
Only safety assessment |
Temporary back and leg pain in the highest dose |
| Petrou et al. [43] |
ALS
(Phase 1/2) |
BM-NTF |
26 |
0 |
48.1, 50.8
(23–65) |
IM
IT
IM + IT |
IM: 2.4–4.8 × 107 cells
IT: 1.0–2.0 × 106 /kg |
1 |
Improvement in the rate of disease progression after 6 months |
Fever,
vomiting,
headache |
| Rushkevich et al. [47] |
ALS |
BM-MSC and neural induced MSC |
10 |
15 |
54.5, 55.0
(37–66) |
IV + IT |
0.5–1.5 × 106/kg
5.0–9.7 × 106 cells
at 5–7-month intervals |
1 or 2 |
Slowing down of the disease
progression after 12 months |
Fever, headache |
| Oh et al. [45] |
ALS
(Phase 1) |
BM |
8 |
0 |
45.7
(29–62) |
IT |
1 × 106/kg at
26-day intervals |
2 |
No efficacy after 6 months |
Fever, pain, headache |
| Kim et al. [48] |
ALS |
BM |
37 |
0 |
52.7, 48.8 |
IT |
1 × 106/kg at
one-month intervals |
2 |
Trophic factors associated with a positive response to treat |
No |
| Mazzini et al. [49] |
ALS (Phase 1) |
BM |
19 |
0 |
20–75 |
Direct injection into spinal cord |
7–152 × 106 cells |
1 |
No long-term adverse effect after nearly 9 years |
No |
2.5. Multiple Sclerosis
Multiple sclerosis is a chronic immune-mediated inflammatory disease in which the immune system progressively destroys the myelin sheath in the central nervous system. This disease can last from a few months to many years. The recent clinical reports using MSCs for the treatment of multiple sclerosis are summarized in
Table 5. Petrou et al. evaluated the optimal safe and effective clinical transplantation of MSCs in patients with active and progressive multiple sclerosis
[50]. In the study, patients were randomized into three groups and treated intrathecally (IT) or intravenously (IV) with autologous BM-MSCs or sham injections. Significantly fewer patients experienced treatment failure in the MSC-IT and MSC-IV groups compared with those in the sham-treatment group. During the 1-year follow-up period, no evidence of disease activity was observed in 58.6% and 40.6% of patients treated with MSC-IT and MSC-IV, respectively, compared with 9.7% in the sham-treated group. MSC-IT transplantation induced additional benefits regarding the relapse rate, and the researchers concluded that the IT administration was more efficacious than the IV administration regarding several parameters of the disease. Furthermore, a safety and feasibility study was completed, focusing on the use of UC-MSCs for treating multiple sclerosis. Twenty subjects were enrolled in the study, and symptom improvements were most notable a month after treatment
[51]. Infusion with MSCs is considered safe and feasible in patients with multiple sclerosis. However, larger studies investigating the number of doses and route of administration are needed to assess potential therapeutic benefits of this technique.
Table 5. Summary of recent clinical trials using MSCs for multiple sclerosis.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Petrou et al. [50] |
Multiple sclerosis
(Phase 2) |
BM |
16,
16 |
16 |
47.6
(37.9–57.3) |
IT
or
IV |
1 × 106/kg at 6-month intervals |
1 or 2 |
Improvement in the course of the disease and comprehensive functional recovery after 3, 6, 12 months.
IT is more efficacious than IV |
No |
| Fernández et al. [52] |
Multiple sclerosis
(phase 1/2) |
AD |
10,
9 |
11 |
44.8
47.8
46.3 |
IV |
1 × 106/kg
or
4 × 106/kg |
1 |
Partial efficacy in the imaging studies and evoked potentials after 12 months |
urinary infection |
| Riordan et al. [51] |
Multiple sclerosis
(phase 1/2) |
UC |
20 |
0 |
41.15 |
IV |
20 × 106 cells at 1–4-day intervals |
7 |
Comprehensive functional recovery after one month |
Headache, fatigue |
| Harris et al. [53] |
Multiple sclerosis
(Phase 1) |
BM MSC -derived neural progenitors |
20 |
0 |
27–65 |
IT |
5.3–10 × 106 cells at 3-month intervals |
3 |
Motor, bladder and comprehensive functional recovery after 3 months |
headache, fever |
| Dahbour et al. [54] |
Multiple sclerosis
(Phase 1/2) |
BM
MSC-CM |
10 |
0 |
34.9
(18–54) |
IT |
93–168 × 106 cells
CM:13–20 mL at 1-month intervals |
1 + 1 |
Comprehensive functional recovery after 12 months |
Pain, headache, fever |
| Llufriu et al. [55] |
Multiple sclerosis
(Phase 2) |
BM |
9 |
0 |
36.8
(23–48) |
IV |
1–2 × 106/kg |
1 |
Improvement in the imaging studies after 6 months |
No |
| Li et al. [56] |
Multiple sclerosis |
UC |
13 |
10 |
41.7, 39.4 |
IV |
4 × 106 cells/kg at 2-week intervals |
3 |
Improvement in the overall symptoms and
fewer incidences of relapse during 12 months |
No |
| Bonab et al. [57] |
Multiple sclerosis
(Phase 2) |
BM |
25 |
0 |
34.7
(23–50) |
IT |
2.95 × 107 cells |
1 |
Improvement or stabilization
in the course of the disease during 12 months |
Fever, nausea, weakness in the lower limbs, headache |
| Lee et al. [58] |
Multiple sclerosis
(Phase 2) |
BM |
16 |
17 |
56.1, 55.8 |
IA (intra-arterial) + IV |
IA: 4 × 107 cells
IV: 4 × 107 cells
at 30-day intervals |
1 + 3 |
Efficacy in preventing the progression of neurological deficits during 12 months |
Small ischemic
lesions |
| Connick et al. [59] |
Multiple sclerosis
(Phase 2) |
BM |
10 |
0 |
48.8
(40–53) |
IV |
1.6 × 106/kg |
1 |
Visual functional recovery after 10 months |
Macular rash, self-limiting infections |
2.6. Parkinson’s Disease
Parkinson’s disease is the common and progressive neurodegenerative disease with major symptoms such as bradykinesia, impaired posture, and tremor. Some studies have reported on the safety and feasibility of MSCs in patients with Parkinson’s disease (
Table 6). Canesi et al. demonstrated the feasibility of BM-MSC in Parkinson’s disease patients. One year after cell infusion, all treated patients were alive, except one, who died 9 months after the infusion for reasons not related to cell administration or to disease progression (accidental fall), and in all treated patients, motor function rating scales remained stable for at least six months during the one-year follow-up
[60]. On the other hand, Carstens et al. showed the efficacy of AD-MSCs in two patients with Parkinson’s disease. After the administration of AD-MSCs, subjective functional recovery after 2 weeks and up to 5 years are observed
[61].
Table 6. Summary of recent clinical trials using MSCs for Parkinson’s disease.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Canesi et al. [60] |
Progressive
supranuclear palsy
(Phase 1) |
BM |
5 |
0 |
60–68 |
IA (intra-arterial) via catheterization |
1.7 (1.2–2.0) × 106/kg |
1 |
Clinical stabilization for at least 6 months during the one-year follow-up |
Transient left hemiparesis |
| Carstens et al. [61] |
Parkinson’s disease
(Case studies) |
AD MSC-derived stromal vascular fraction |
2 |
0 |
72, 50 |
Facial and nasal transplantation |
6.0 × 107 cells |
1 |
Subjective functional recovery after 2 weeks and up to 5 years |
No |
2.7. Traumatic Brain Injury
Traumatic brain injury is one of the major serious diseases that threaten human life and health, causing traffic accidents, collisions with hard objects, and falls from high places. With improving medical technology, the survival rate of patients with traumatic brain injury has increased significantly. However, the prognosis for patients with severe TBI remains poor, such as disturbance of consciousness and motor disorder. The recent clinical reports using MSCs for the treatment of traumatic brain injury are summarized in
Table 7. Wang et al. showed the results of a phase 2 clinical trial using UC-MSCs for traumatic brain injury patients
[62]. Forty patients with sequelae of traumatic brain injury were randomly assigned to the stem cell treatment group or the control group, and UC-MSCs administration improved the neurological function and self-care in patients after 6 months. On the other hand, Tian et al. explored the clinical therapeutic effects and safety of autologous BM-MSCs therapy for traumatic brain injury by lumbar puncture
[63]. The results showed improvement in the function of brain in the form of post-therapeutic improvements in consciousness and motor functions. In addition, they showed the age of patients and the time elapsed between injury and therapy had effects on the outcomes of the cellular therapy, and no correlation was found between the number of cell injections and improvements.
Table 7. Summary of recent clinical trials using MSCs for traumatic brain injury.
| Reference |
Disease |
Source |
Number |
Mean Age (Range), Year |
Route of Administration |
Number of Cells |
Number of Treatments |
Results |
Adverse Events |
| Trial |
Control |
| Wang et al. [62] |
Traumatic brain injury
(Phase 2) |
UC |
20 |
20 |
27.5 ± 9.4
28.6 ± 10.1 |
IT |
6.0 × 107 cells |
4 |
Comprehensive functional recovery and improvement in the ADL
after 6 months |
Mild dizziness, headache |
| Tian et al. [63] |
Traumatic brain injury |
BM |
97 |
0 |
- |
IT |
3.0–5.0 × 106 cells |
1 |
Improvement of consciousness and motor function after 14 days |
No |