2.1. Glucotoxicity
CD36 exerts fundamental biological functions at the cellular and tissue levels in multiple homeostatic and pathological processes by its distinct binding sites. Pancreatic β-cells play a central role in regulating glucose metabolism to sustain energy homeostasis by mediating a balance between insulin, an anabolic hormone, and glucagon, a catabolic hormone. Pancreatic β-cells require suitable sensors and signaling molecules that are integrated to modulate insulin secretion and maintain homeostasis. However, type 2 diabetes (T2D) is based on the inability of pancreatic β-cells to sustain a compensatory secretory response, leading to insulin secretory dysfunction and the pathogenesis of T2D. CD36 is the most generously expressed transporter among fatty acid transporters in human islets. It is located in the plasma membrane and co-localizes with insulin granules
[12]. Interestingly, CD36 was shown to traffic between intracellular compartments and the cell surface in a vesicle-mediated process
[13]. It has been well established that glucose potentiates fatty acid-induced β-cell death via apoptosis
[14][15]. Similarly, the overexpression of CD36 in β-cells increases the uptake of fatty acids and leads to metabolic and functional dysfunction
[16]. We also reported that glucotoxicity influences pancreatic β-cell dysfunction by increasing the influx of free fatty acids (FFAs) via CD36
[17]. To evaluate the mechanisms by which glucotoxicity affects β-cell dysfunction, we investigated CD36 expression and trafficking in β-cells and observed that Rac1, a small Rho family protein, displays increased glucose-mediated CD36 expression on the membrane surface in pancreatic β-cells
[18]. The importance of Rac1 signaling in early-phase insulin secretion was previously demonstrated in β-cell-specific Rac1-deficient mice via the inhibition of F-actin depolymerization
[19][20]. Glucose stimulates the recruitment of insulin granules to the cell membrane through actin remodeling, which is necessary for glucose-stimulated insulin secretion
[21][22]. The F-actin function is coupled to SNARE-associated proteins such as syntaxin 1, syntaxin 4, and SNAP-25 in β-cells, and many F-actin-binding proteins interact with the SNARE machinery
[21][23][24][25]. Several studies have shown that deficiencies in SNARE proteins are likely caused by high glucose and might contribute to cell dysfunction in disease states
[26][27][28]. More considerations concerning SNARE protein function are discussed in the review article by Gaisano et al.
[29]. Recently, it was shown that CD36 overexpression attenuates insulin secretion in human islets through the reduction of the exocytotic genes Snap25 and Vamp2, resulting in a decreased number of docked granules. It was further demonstrated that CD36 overexpression attenuates insulin signaling, resulting in the accumulation of the transcription factor FoxO1 in the nucleus as a potent transcriptional repressor of exocytotic genes. Interestingly, the inhibition of CD36 was shown to upregulate exocytotic gene expression in human islets, improving granule docking and resulting in increased insulin secretion without affecting insulin content
[30]. Further research is required to identify the roles of CD36 in exocytotic gene function as well as whether F-actin function is involved in suppressing insulin secretion by CD36. Such studies will provide greater insights into the mechanisms of how CD36 induces metabolic dysfunction in pancreatic β-cells.
On the other hand, evidence suggests that hyperglycemia leads to the generation of reactive oxygen species (ROS), resulting in increased oxidative stress in β-cells
[31][32]. The activation of Rac1 increases the production of oxidants, such as H
2O
2, via the activation of NADPH oxidase (NOX), which might trigger oxidative stress linked to β-cell death in T2D
[33]. It was previously observed that CD36 deficiency reduces NOX activity and attenuates obesity-associated oxidative stress in the heart
[34]. We also observed that Rac1 mediates NOX activity, leading to an increase in CD36 at the plasma membrane and that Rac1 and NOX inhibition can abrogate CD36 downstream signaling damage in response to high glucose
[18]. However, it remains unknown how CD36 translocation to the plasma membrane is detected after Rac1-NOX activation by high glucose. One possible explanation may involve the palmitoylation of CD36 by supraphysiologic glucose levels. High-glucose-induced Rac1-palmitoylation has been suggested to be a driving force behind the activation of NOX, which in turn would alter the localization of Rac1 remodeling in diabetic retinopathy
[35]. Nonetheless, a current topic of research is to elucidate how protein palmitoylation influences the function of proteins under high glucose conditions in pancreatic β-cells. It should be noted that increasing ROS production can alter cellular dysfunction stimulated by the activation of stress kinases by changing the balance of antioxidant enzymes. Previous findings also reported that CD36 altered cellular signaling under metabolic stress conditions by downregulating the redox-sensitive nuclear factor Nrf2 via Fyn kinase in murine vascular smooth muscle cells
[36]. In addition, CD36 signaling in response to scavenger ligands leads to the activation of Src and MAPK family kinases, such as Lyn and c-Jun
N-terminal kinase (JNK) in macrophages and platelets, whereas Fyn and p38 are the primary mediators of endothelial cells
[37][38][39]. We also reported that Rac1-CD36 signaling by high-glucose-induced JNK and p38MAPK activation and the inhibition of CD36 inhibition blocks high-glucose-induced oxidative stress. Lots of evidence has suggested that ER stress is linked to insulin resistance, and pancreatic β-cell ER expansion was detected in patients with T2D
[40][41]. Cells activate adaptive, self-protective mechanisms in response to ER stress, which are collectively referred to as the ER stress response (also named UPR). These include enhanced ER size, increased ER folding capacity through the manipulation of chaperones and foldases, decreased biosynthetic load, and the increased clearance of unfolded proteins through the stimulation of ER-related degradation. When these systems fail to alleviate the stress, apoptosis is triggered. Subsequent work from our lab has demonstrated that chronic glucose exposure or thapsigargin treatment induces ER stress through the reduced expression and activity of insulin and PDX1 with CD36 induction. Inhibition of CD36 in β-cells by metformin treatment or by using CD36 siRNA was shown to prevent the generation of ER stress markers and stress kinase activation
[42]. It is clear that signaling related to CD36 regulation and its dynamics has impacts on oxidative stress, and understanding this linkage warrants further investigation. Given that CD36 signaling is related to numerous pathological events, β-cell CD36 downstream targets need to be further investigated.
2.2. Lipotoxicity
Hyperglycemia with elevated FFAs plays a significant role in insulin resistance and β-cell dysfunction in T2D
[43][44][45]. Many studies have shown that glucose enhances fatty acid-induced β-cell death via apoptosis
[14][15]. Fatty acid–glucose balance is essential for maintaining normal β-cell function, but lipotoxicity-induced β-cell dysfunction occurs with increased ROS, ceramide and nitric oxide levels, and mitochondrial perturbations
[46][47]. Studies in Zucker diabetic fatty (ZDF) rats, an obesity-induced diabetic animal model, confirmed FFA-induced ceramide accumulation leading to β-cell apoptosis
[48]. Another study demonstrated that superoxide production was elevated in islets isolated from Zucker lean fatty (ZLF) and Zucker diabetic fatty (ZDF) rats in the presence of glucose
[49]. The resting superoxide content of ZDF rat islets was higher than that of Zucker lean control islets and was accompanied by the alteration of mitochondrial morphology. The FFA-induced formation of ceramide also induces the generation of ROS and DNA fragmentation
[50]. Collectively, oxidative stress and mitochondrial dysfunction result in endogenous antioxidant impairment. However, plasma from patients with obesity and T2D shows enhanced levels of ceramides, which may serve as biomarkers for the diagnosis and treatment of obesity and diabetes
[51][52][53]. Based on its role in FFA uptake, our lab showed that CD36 might promote ceramide-induced β-cell dysfunction by the Src-mediated tyrosine phosphorylation of Vav, a guanine nucleotide exchange factor (GEF), and also elevate metabolic pathways via its GEFs activity
[54]. Evidence suggests that saturated fatty acids impair insulin secretion and induce insulin resistance via Src signaling in T2D
[55]. Accordingly, we hypothesize that the induction of CD36 could be recapitulated in cells with functional vav tyrosine phosphorylation by Src promoting Rac1 signaling that generates ROS by NOX. Our results indicate that CD36-mediated Src-Vav activation is necessary for optimal Rac1-NADPH-induced superoxide production. To determine whether or not CD36 is linked to Vav-Rac1-NOX activation, we performed pharmacological inhibition of Src activity or CD36 siRNA, which significantly reduced ceramide-induced RAC1-NOX and inhibited ROS formation
[56]. Holzer et al. demonstrated that saturated fatty acids stimulate stress-signaling activation by Src via transfer to a membrane micro domain
[57]. In addition, the FFA-mediated Src-dependent Vav phosphorylation coordinates the engagement of Rac1-NOX-JNK signaling, which contributes to insulin resistance, obesity, and the production of inflammatory cytokines
[58][59]. In podocytes, CD36-dependent uptake of palmitic acid leads to impaired mitochondrial energy metabolism, the alteration of mitochondrial and ER morphology, increased levels of mitochondrial ROS, the depolarization of mitochondria, ATP depletion, and apoptosis
[60][61][62].
A recent study showed that p66Shc mediates lipotoxicity-induced impaired metabolic changes that promote pancreatic β-cell dysfunction and apoptosis in diabetes
[32]. Earlier studies have suggested that p66Shc serine36 phosphorylation by JNK leads to ROS production and cell death
[63]. Activated JNK combined with p66Shc serine36 phosphorylation activation to induce mitochondrial ROS in response to CD36 signaling promote cellular dysfunction. Cells lacking this pathway, as a consequence of CD36 inhibition, significantly block ceramide-induced β-cell dysfunction. Evidence points to CD36 signaling-generated H
2O
2, which promotes cysteine sulfenylation, a post-translational modification important to the augmentation of platelet activation and aggregation
[64]. Peroxiredoxins, a thioredoxin-dependent peroxide reductase family of antioxidant proteins, catalyze the reduction of both hydrogen peroxide and alkyl peroxides to water and their corresponding alcohols
[65][66]. The expression of peroxiredoxin-3 (PRDX3) is restricted to β-cells in pancreatic tissue. The oxidation of peroxidase cysteine to sulfonic acid (peroxiredoxin-SO3) promotes the accumulation of oxidized PRDX3 in mitochondria, which favors mitochondrial permeability transition pore (MPTP) opening and mitochondrial swelling
[67]. Importantly, ceramide-induced sulfenylation is reduced in the presence of CD36 inhibition, which is consistent with a CD36-dependent mechanism. However, reduced PRDX3 is regulated by the thioredoxin–thioredoxin reductase system. Accordingly, we hypothesize that the inhibition of thioredoxin could be recapitulated in cells with functional thioredoxin-interacting protein (TXNIP) by preventing peroxiredoxin-3 activity in response to ceramide. We observed that TXNIP translocates to mitochondria and inhibits the antioxidative protein thioredoxin in response to ceramide. Moreover, ceramide-induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation has been shown to increase TXNIP expression in β-cells
[68]. This finding suggests that CD36 plays an important role in the initiation of oxidative stress induced by ceramide under conditions of β-cell failure. Thus, exploration of CD36 warrants further investigation.
Under normal conditions, mitochondria in β-cells persistently undergo fusion and fission. These processes may function to refute the negative impacts of the long-term presentation of β-cells to palmitate under high glucose conditions, causing mitochondrial fragmentation and impeding network dynamics by abolishing fusion and fission activity
[69]. There are two powerfully contradictory processes that determine mitochondrial shape and morphology: fusion and fission. The ablation of both fusion and fission produces a significant effect on the progression of cells to apoptosis
[70]. It has been reported that the mitochondria of β-cells from Zucker diabetic rats are divided, suggesting an imbalance in the mitochondrial fusion and fission process
[49]. The exposure of β-cells to high-fat glucose conditions causes the discharge of Ca
2+ from the ER to the cytoplasm, driving a rise in the cytosolic Ca
2+ concentration that reflects expanded mitochondrial Ca
2+ uptake. Increased mitochondrial Ca
2+ uptake improves local buffering capacity and the discharge of proteins competent in apoptosis induction. Hence, Ca
2+ activates the phosphatase calcineurin, which dephosphorylates and inactivates dynamin-related protein 1 (Drp1), a master controller of mitochondrial fission
[71]. It can be assumed that crosstalk between the ER and mitochondria may promote cellular commitment to apoptosis through Ca
2+. Recently, the presence of inositol trisphosphate receptor (IP3Rs) has been implicated in proapoptotic Ca
2+ transfer between the ER and mitochondria
[72]. An important remark is that Akt restrains the ER-to-mitochondria Ca
2+ exchange by means of IP3R3 and ensures against Ca
2+ intervened apoptosis
[73]. Interestingly, CD36 was found to be overexpressed in obese diabetic islets and suppressed the insulin-signaling PI3K/AKT pathway, as well as its downstream transcription factors
[30]. These effects result in ER-mitochondrial reprogramming, which contributes to the development of β-cell death and failure. The different pathways enacted by CD36 require further confirmation of the precise roles of CD36 signaling pathways in β-cell failure.
2.3. OX-LDL and Amyloid Deposition
CD36 can generate cell-specific reactions to multiple ligands through the binding of context-specific binding partners that contribute to the development of β-cell dysfunction. As described above, it has been shown that ER stress is connected to insulin resistance in diabetes, and, conjointly, an expansion of ER was recognized in β-cells from patients with T2D
[39][40]. Oxidized-LDL (oxLDL)-induced ER stress activation is coupled with oxidative stress, leading to β-cell dysfunction and death
[74]. It has been shown that oxLDL induces β-cell dysfunction and apoptosis via the activation of ROS and that radical lipid hydroperoxides contribute to JNK activation
[75][76][77]. However, the downstream mechanism by which JNK leads to apoptosis is not yet clear, and the crosslink between oxLDL and CD36 may promote cellular commitment to apoptosis through JNK enactment. A previous study reported that oxLDL intervenes with the JNK-dependent phosphorylation of p66Shc in endothelial cells, which contributes to oxidative stress and the atherogenic progression
[78], Thus, we cannot preclude a role for PRDX3 oxidation in CD36 signaling. In this way, p66Shc can result in the overproduction of H
2O
2, which in turn can react with PRDX3 to cause toxic mitochondrial dysfunction and apoptosis.
Regarding the molecular mechanisms involved, CD36 overexpression partners with the increased uptake of oxLDL without exerting additive effects on oxLDL toxicity
[79]. Evidence suggests that CD36 causes a mitochondrial metabolic switch from oxidative phosphorylation to superoxide generation in reaction to oxLDL, which subsequently promotes NF-κB activation and the generation of pro-inflammatory cytokines
[80]. Hence, redox status is subordinate on the degree to which a cell’s components exist in an oxidative state, whereby a reducing environment inside cells can prevent oxidative stress. oxLDL also initiated the ASM/ceramide signaling pathway, which is involved in macrophage apoptosis via the ER stress pathway
[81]. However, the downstream targets of oxLDL in β-cells are not well known, and additional studies are needed.
On the other hand, β-cells have a lower abundance of antioxidant defense enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx)
[82][83][84]. As such, the administration of antioxidant supplements can increase the defense capacity of islet cells to cope with oxidative stress
[85]. Vitamin E is a redox-active natural compound that downregulates levels of ROS under different experimental conditions
[86][87][88][89]. Interestingly, vitamin E reduces the uptake of OX-LDL by inhibiting CD36 expression via the PPARγ signaling pathway
[90][91][92]. Furthermore, vitamin E facilitates the activation of PI3Kγ/AKT, leading to increased VEGF expression as well as elevation of cell survival and angiogenesis via its ability to increase tissue remodeling
[93]. In addition, genetic data indicate that VEGF is a major regulator of islet vascularization and the revascularization of transplanted islets, and reduced beta-cell VEGF expression impairs glucose-stimulated insulin secretion
[94]. Furthermore, the addition of vitamin E induces insulin secretion and islet-cell survival and functionality by enhancing PDX1, a master regulator of insulin gene expression
[95][96]. Thus, the elevated expression of CD36 in beta cells exposed to elevated OX-LDL results in increased ROS expression that could induce discrete oxidative stress. Therefore, we suggest that the vitamin E-induced elevation of insulin expression may be mediated by CD36 inhibition, which may explain, at least in part, the reported protection against oxidative stress. Further studies are needed to elucidate the relationship between CD36, vitamin E, and OX-LDL in the pancreatic β-cell dysfunction.
Among the variety of proapoptotic factors present in pancreatic β-cells, islet amyloid polypeptide (IAPP) is thought to play a crucial role in β-cell apoptosis. The proapoptotic effects of IAPP are mediated through a complex sequence of signaling events that lead to defects in mitochondrial dysfunction, autophagy, local inflammation, oxidative stress, cytokine production, and the enactment of signaling pathways driving to apoptosis
[97][98][99][100]. Interestingly, CD36 can create a solid pro-inflammatory reaction through its interaction with secreted amyloid-beta 1–42 (Aβ) in macrophages
[101]. However, the presence of this CD36-dependent pro-inflammatory signaling hub within the pancreatic β-cells has not been studied and warrants further investigation (
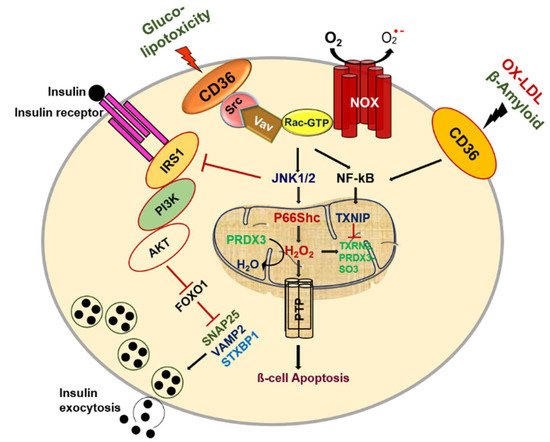
Figure 1).
Figure 1. CD36 signal transduction in pancreatic β-cell dysfunction.