1000/1000
Hot
Most Recent

Ferrofluids (FFs) are stable and quite homogeneous colloidal dispersions of ferromagnetic nanoparticles in a solvent referred to as the carrier.
Ferrofluids (FFs) are stable and quite homogeneous colloidal dispersions of ferromagnetic nanoparticles in a solvent referred to as the carrier. These materials have the properties of both of their components: (i) the magnetic nanoparticles (MNPs), which confer a paramagnetic behavior to the suspension; and (ii) the carrier, which is responsible for the fluidic nature of the material [1]. It is important to highlight that a liquid dispersion of MNPs can be considered as FF only if it presents enough uniformity to be a magnetic liquid by itself, and without observing the separation of those particles from the carrier when an external magnetic field is applied [2].
MNPs used to prepare FFs are generally made of magnetite (Fe3O4), maghemite (γ-Fe2O3), or either Fe (II)/Fe (III) compounds, the magnetic dipolar moment of which can be aligned parallelly to the dipolar moment of an external magnetic field [3]. Traditionally, MNPs used for the preparation of FFs are loaded at low content in the carrier, between 3% and 10% of the FF. Besides, MNPs with a particle size around 10 nm are preferred to form FFs [2]. Nevertheless, the size of the particles should be taken simply as an illustrative parameter, as MNPs with higher diameters have also been used to prepare FFs [4]. Regarding the carrier, it is the main component in the FF (90–97%), providing its liquid nature (despite the presence of small amounts of solid particles in suspension). It is also responsible for most FFs properties. The proper selection of the carrier allows the preparation of tunable FFs. A wide variety of solvents have been utilized as FFs carriers, either conventional solvents (organic solvents—mainly alcohols—and water) [5][6] and non-conventional solvents, including ionic liquids (ILs) [7], deep eutectic solvents (DESs) [8], and supramolecular solvents (SUPRASs) [9].
The stability of FFs can be clearly affected by the agglomeration of the MNPs, due to Van Der Waals interactions and magnetic attractions, among others. Thus, FFs need to incorporate a stabilizer or a dispersing agent to prevent such aggregation [10]. The stabilizer can be directly incorporated into the initial mixture of MNPs plus carrier, or the stabilization of MNPs can be accomplished by their surface modification through functionalization. The stabilization can take place through two different mechanisms of repulsion: (i) ionic repulsion and (ii) steric repulsion. Ionic stabilizers form a charged layer over the surface of MNPs, causing repulsion forces between the MNPs as they have the same electrostatic charge. On the other hand, steric repulsion is produced by the formation of a thicker layer that moves MNPs away from each other due to the steric hindrance. This layer is formed into two steps: the first stabilizer layer is attached by chemisorption and followed by a second layer that is attached by physisorption on the surface of MNPs [1]. Surfactants are the most widely used stabilizers in FFs due to their high molecular weight, which, in most cases, favors the establishment of steric repulsions, and, in the case of ionic surfactants, through the incorporation of the ionic repulsion. Oleic acid, lauric acid, palmitic acid, and stearic acid are some of the most representative surfactants used as stabilizer agents [11]. Some of the solvents used as FFs carriers have also been explored as ionic stabilizers, with ILs being the most popular solvents for this role [12].
The main preparation procedure of FFs is quite simple: the direct mixing of the precursors (stabilized MNPs and carrier, or MNPs, stabilizer, and carrier) for a certain time, to ensure the formation of a quite homogeneous colloidal suspension. Ultrasonic energy is the most common assistance method used to ensure such uniform and energic mixing [5][9][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28]. In any case, the preparation of any FF clearly requires the synthesis of the MNPs, and, in several cases, the surface modification of the MNPs, and/or the synthesis of the carrier solvent if it is not commercially available.
If considering green issues, water-based FFs are undoubtedly the easiest and most direct and environmental-friendly types, while being (in general) biocompatible. In any case, the green label cannot be exclusively linked to their water-based nature, and indeed green aspects related to the synthetic procedure of the MNPs need to be also considered. Thus, it is important to consider if hazardous chemicals have been used as precursors, or if an inert atmosphere and/or thermal treatments at high temperatures for long times have been also required [29].
The most important feature of FFs is their magnetic susceptibility, which gives them an impressive magnetic-responsive behavior. The application of an external magnetic field can induce several modifications in the properties of FFs, making them smart and tunable materials with great potential in a wide variety of disciplines. Indeed, the application of magnetic fluids is not a recent trend in magnetochemistry [30] and biomedicine [31], among other fields. However, the use of FFs as extraction materials in sample preparation is a relatively new topic.
The use of FFs within analytical chemistry has not been extensively consolidated, and indeed there is still controversy concerning mechanisms of FF-based extractions. To sum up, there is no consensus on the classification of the technique into the different existing analytical extraction methods [32]. From an operational point of view, in general, the extraction procedure based on FFs requires a dispersion of the as-prepared FF into the sample, followed by the application of an external force to ensure a strong mixing sample-FF. Once the analytes have been partitioned from the sample to the FF, the FF is decanted using an external magnet. Finally, as it is not possible to directly inject the FF into a chromatographic system (the most common technique in the reported applications) without damaging it, the FF is dispersed in a few µL of a solvent to perform a desorption step of the retained analytes. Figure 1 shows a general scheme of the most common extraction procedure followed when using FFs.
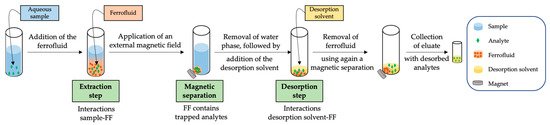
Figure 1. General scheme of the most common operational procedure followed in ferrofluid (FF)-based analytical extraction methods.
This extraction procedure has been identified by several authors as magnetic micro-dispersive solid-phase extraction (m-µ-dSPE), because the operational procedure resembles that of m-µ-dSPE, including both extraction and desorption steps [6][15][21][22][23][24][25][26][33][34][35][36], while other studies have included FF-based extractions within magnetic dispersive liquid–liquid microextraction (MDLLME) methods [5][8][9][13][16][17][18][27][37][38][39][40]. These two different classifications rely on the following considerations: (i) the main component is a liquid and the content of solid MNPs is almost negligible, and, thus, it can be classified as liquid-based; and (ii) the partition of the analytes in the extraction step takes place from the liquid sample to the solid magnetic micro-extractant phase, and, thus, it can be classified as solid-based. Independent of the classification, it is clear that the extraction mechanism comprises a synergetic effect between the role of the MNPs, which confer the magnetic nature to the FF (allowing the magnetic separation), and the role of the liquid carrier as the main promoter of the interactions between the analytes and the FF. Assuming that this important role of the carrier and the solvation effects of the FF given its (mostly) liquid nature, FF-based extraction procedures would be better classified as magnetic liquid-phase microextraction (MLPME).
The first FF used with extraction purposes was reported in 2010 by Shi et al. [5]. It was prepared by simple dispersion of 10 mg of silica-coated Fe3O4-based MNPs in 100 µL of 1-octanol as the carrier, under sonication. In this study, 28 mg of FF were added to 20 mL of an aqueous sample under agitation. After 20 min of extraction time, the FF containing the trapped analytes was separated by using an external magnet. Finally, the extracted analytes were desorbed using 100 µL of acetonitrile under sonication for 2 min. Then, the eluate was directly injected in a gas chromatograph (GC) equipped with a mass spectrometer (MS) for the determination of polycyclic aromatic hydrocarbons (PAHs). The incorporation of the FF reduced the extraction time needed, as any magnetic-based extraction method, avoiding centrifugation steps, and permitting a solvent-saving sample preparation approach.
Since then, a wide variety of analytical applications have arisen using FFs with different solvents as carriers. Undoubtedly, the chemical and physical properties of the carrier solvent exert an important influence on the extraction performance. Thus, the proper selection of the carrier is decisive for the design of the FF and its intended application in analytical sample preparation procedures.
In this section, we propose to classify the FFs as a function of the nature of the carrier solvent. The main solvents used as carriers of FFs in sample preparation applications are (i) alcohol and water, (ii) ILs, (iii) DESs, and (iv) SUPRAS. Figure 2 shows the number of studies for each type of FF according to this classification. It is important to highlight that this review article focuses on those articles that clearly incorporate FFs in analytical studies. Table 1 contains a summary of those analytical applications using FFs [5][6][7][8][9][13][14][15][16][17][18][19][20][21][22][23][24][25][26][28][33][34][35][36][37][38][39][40][41][42][43][44]. As it can be observed, different analytes have been determined in a wide variety of samples, using all the types of FFs. Thus, the intended analytical application is not the main criterium to select a specific type of FF in the studies reported. In general, FFs are selected by their carrier, and each study benefits from the features of the carrier solvent, as is described in detail in this section for each type of FF.
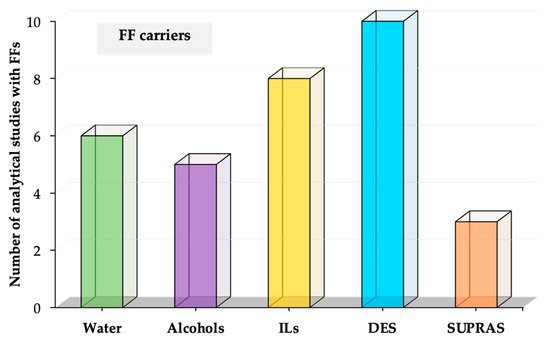
Figure 2. Number of analytical applications using FFs, with the type of FF classified as a function of the different carriers used for their formation.
Table 1. Main analytical features of the reported FF-based extraction applications.
| Nanoparticle/Carrier | Amount of Ferrofluid (µL) | Analytes (Number) | Sample/Volume (mL) | Analytical Technique | LOQ | RSDmax * (%) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Water-based FFs | ||||||||
| γ-Fe2O3@citrate/water | – | metals (4) | water (–) | ICP-AES | – | – | – | [35] |
| Fe3O4@decanoic acid/water | 1000 | drugs (2) | water, plasma and urine (5) | HPLC-UV | 10 µg·L−1 | 8.4 | 89.6–97.4 | [6] |
| Fe3O4@oleic acid/water | 750 | aflatoxins (4) | MeOH/water extract of food (18) | spectrofluorimetry | 21 ng·L−1 ** | 4.6 | 91.6–99.6 | [41] |
| Fe3O4@oleic acid/water | 500 | aflatoxin (1) | MeOH/water extract of food (18) | spectrofluorimetry | 13 ng·L−1 ** | 4.3 | 91.3–99.5 | [42] |
| Fe3O4@decanoic acid/water | 1000 | drugs (3) | water and urine (20) | GC-FID | 10 µg·L−1 | 9.4 | 90.0–104 | [36] |
| Fe3O4@oleic acid/water | 500 | drug (1) | plasma (5) | spectrofluorimetry | 0.21 µg·L−1 | 1.5 | 93.5–102 | [43] |
| Alcohol-based FFs | ||||||||
| Fe3O4@oleic acid/1-heptanol | 100 | phenolic compounds (4) | beverages (20) | HPLC-DAD | 0.66 µg·L−1 ** | 7.3 | 83.7–106 | [13] |
| Fe3O4@SiO2/1-octanol | 28 (mg) | PAHs (16) | water (20) | GC-MS | 0.19 µg·L−1 | 12 | 59.2–93.3 | [5] |
| Fe3O4@SiO2/1-octanol | 200 | metal (1) | soil and road dust after acid digestion (50) | FAAS | 0.35 µg·L−1 ** | 3.3 | 95.2–103 | [33] |
| Fe3O4@SiO2/1-octanol | – | metal (1) | nail, hair, cabbage and iron after acid digestion (150) | FAAS | 0.21 µg·L−1 ** | 3.2 | 95.0–105 | [14] |
| Fe3O4@poly(β-CD-IL)/1-octanol | – | PAHs (7) | water, beverages and ACN extract of food (15) | GC-FID | 0.21 µg·L−1 | 8.9 | 80.5–116 | [37] |
| IL-based FFs | ||||||||
| Fe3O4@SiO2/[C4MIm]+[BF4]− | 25 mg | metal (1) | water and food after wet digestion (150) | FAAS | 0.11 µg·L−1 ** | 4.0 | 97.5–103 | [34] |
| Fe3O4@GO/[C4MIm]+[BF4]− | – | metal (1) | water, and vegetable and tobacco after acid digestion (250) | FAAS | 0.12 µg·L−1 ** | 1.4 | 98.2–101 | [21] |
| Fe3O4@GO/[C4MIm]+[BF4]− | – | metals (2) | water (100) | GFAAS | 26 ng·L−1 | 6.5 | 83–117 | [22] |
| Fe3O4@SiO2/[C6MIm]+[BF4]− | – | metal (1) | waters, and road dust and food after acid digestion (200) | FAAS | 1.7 µg·L−1 ** | 1.34 | 96.0–118 | [23] |
| Fe3O4@SiO2/[C6MIm]+[BF4]− | – | metal (1) | water, beverages, and food after acid digestion (200) | FAAS | 0.32 µg·L−1 ** | 2.6 | 95.5–105 | [24] |
| Fe3O4@SDS/[C6MIm]+[BF4]− | – | dye (1) | water and EtOH extract of food (175) | UV-Vis | 2.5 µg·L−1 ** | 2.9 | 99.0–109 | [25] |
| BaFe/[C6MIm]+[NTF2]− | 50 | pesticides (5) | beverages (10) | HPLC-DAD | 0.53 µg·L−1 ** | 5.3 | 85.1–99.6 | [7] |
| Fe3O4@CQD/[C8MIm]+[PF6]− | – | phenolic compounds (4) | water and beverage (20) | HPLC-DAD | 0.17 µg·L−1 ** | 4.1 | 94.5–102 | [26] |
| DES-based FFs | ||||||||
| Fe3O4@SiO2/ChCl:EG | 250 | drug (1) | plasma and urine (10) | HPLC-UV | 2.5 µg·L−1 ** | 6.2 | 92.8–98.4 | [15] |
| Fe3O4@oleic acid/menthol:HAc | 60 | drug (1) | urine (10) | HPLC-UV | 4.6 µg·L−1 | 4.6 | 80.3–97.4 | [16] |
| Fe3O4@oleic acid/menthol:octanoic acid | 150 | drug (1) | urine, blood plasma, and beverage (10) | HPLC-UV | 8.5 µg·L−1 | 5.7 | 86.7–97.5 | [17] |
| Fe3O4@SiO2/PCHCl:menthol:decanoic acid | 90 | PAHs (16) | ACN extract of food (6) | GC-MS | 0.27 µg·kg−1 | 10 | 73–92 | [18] |
| Fe3O4@Aliquat336/ChCl:stearic acid | 76 | PAHs (16) | saliva and urine (5) | GC-MS | 0.22 µg·L−1 | 9 | – | [19] |
| Fe3O4@Aliquat336/ChCl:stearic acid | 85 | pesticides (6) | beverages (30) | GC-MS | 40 ng·L−1 | 5.3 | 89.0–105 | [20] |
| Fe3O4@Aliquat336/ChCl:stearic acid | 225 | pesticides (7) | beverages (30) | GC-MS | 24 ng·L−1 | 7.9 | 82–94 | [38] |
| Fe3O4@SiO2/Et4N+:EG | 150 | flavonoids (1) | beverages (10) | HPLC-UV | 3.0 µg·L−1 | 3.8 | 82–98 | [8] |
| Fe3O4@clay/menthol:decanoic acid | 50 | explosives (11) | water and ACN extract of soil (10) | HPLC-UV | 0.91 µg·L−1 ** | 8 | 99–104 | [44] |
| Fe3O4@SDBS/menthol:decanoic acid | 200 | metal (1) | water (50) | FAAS | 2.2 µg·L−1 | 2.6 | 96–99 | [39] |
| SUPRAS-based FFs | ||||||||
| Fe3O4@CLDH(Zn-Fe)/1-dodecanol:toluene | 8 | pesticides (2) | beverages (–) | GC-FID | 2 µg·L−1 | 6.5 | 85.0–96.6 | [28] |
| Fe3O4@oleic acid/decanoid acid:Bu4N+ | 2000 | drug (1) | plasma and urine (30) | spectrofluorimetry | 0.2 µg·L−1 ** | 2.9 | 94.0–106 | [9] |
| Fe3O4@oleic acid/decanoid acid:Bu4N+ | 2000 | pesticides (3) | water and beverages (10) | HPLC-UV | 0.35 µg·L−1 ** | 5.7 | 92.2–110 | [40] |
* RSDmax as maximum relative standard deviation value reported in the study (in %). ** Limit of detection. For the definition of the abbreviations, please refer to the list of abbreviations at the end of the article.
Alcohols were the first group of solvents used as carriers to prepare FFs in analytical chemistry extractions, given their availability and also their wide use as conventional organic solvents in many sample preparation methods [13][14][33][37]. Besides, the polar character of these solvents ensures a high number of interactions with polar and ionic analytes, including dipole-dipole and ion-dipole forces. Among the studied alcohols, 1-octanol is the most commonly used as a carrier [14][33][37]. This carrier has been mixed with Fe3O4 coated with silica [14][33], and with a poly (cyclodextrin-ionic liquid) [37], to prepare the alcohol-based FFs. In these studies, the surface functionalization of MNPs was the tool selected to prevent their agglomeration. An interesting study by Yang et al. used different alkyl chain alcohols (from C4 to C10) as carrier solvents and oleic acid-coated MNPs. It was observed that the extraction efficiency increases with the length of the alkyl chain from C4 to C7, being maximum when using 1-heptanol. For longer chains, the dispersion of the alcohol-based FF on the sample was hindered due to more hydrophobic interactions with phenolic compounds as target analytes [13].
As a step forward to improve the sustainability of FF-based extraction methods, water has been also explored as a carrier [6][35][36][41][42][43], and indeed water has gradually replaced alcohols as the most widely used carrier. Most water-based FFs reported include oleic acid-coated Fe3O4-based MNPs [41][42][43], although decanoic acid has also been used as stabilizing agent [6][36]. It is interesting to mention that Ngomsik et al. reported the use of a water-based FF composed of citrate-coated maghemite-based MNPs (γ-Fe2O3) and water [35], instead of the common use of magnetite as the core of the MNPs.
The size of the MNPs within alcohol-based and water-based FF applications has evolved during the last decade. Thus, the diameter of MNPs range from 50 nm [33] to 90 nm [5] when alcohol is used as the carrier, whereas water-based FFs are made of smaller MNPs (less than 20 nm) [43]. The first reported FF was the one with the largest MNPs size [5], and, since then, the reported sizes of MNPs have been decreasing until reaching the most common size of 10 nm, which is now specified in the generic definition of a FF.
Extraction methods using alcohol-based and water-based FFs as extractants have benefited from the highly stable interactions between analytes and carriers. These favored interactions also justify the short extraction times of applications based on these FFs. The most common assistance for extraction is vortex or stirring [6][13][36][37][41][42][43], with extraction times ranging from 2 min [41][42][43] to 15 min [36]. In several studies, the extraction only requires a few seconds without needing any assistance [14], or the extraction time is not reported, as it is practically an instantaneous process [33]. With respect to the desorption step, the most common organic solvents used are methanol (MeOH) [6][13] and acetonitrile (ACN) [41][42]. The amount of alcohol-based and water-based FFs used in analytical sample preparation applications ranges between 500 µL [42][43] and 1 mL [6][36].
Some studies have combined the properties of FF with other extraction procedures to accomplish a positive synergetic effect on the overall extraction efficiency [41][42][43]. These methods comprise the first DLLME procedure in which an alcohol is used as the extractant, followed by an in situ formation of the FF containing the extracted analytes.
The most common analytes extracted with alcohol-based and water-based FFs include metals [14][33][35] and organic compounds, such as PAHs [5][37], drugs [6][36][43], and phenols [13], from a wide variety of samples, such as water [6][35][36][37], food [13][37][41][42], and biological samples [6][14][36][43]. When solid samples are analyzed, there is, in general, a previous extraction step, or even a digestion step, and the aqueous extract (or digested sample) is then subjected to the FF-based extraction [33][41].
With respect to the most used analytical techniques, flame atomic absorption spectroscopy (FAAS) has been used for the determination of metals [14][33], whereas spectrofluorimetric [41][42][43] and different chromatographic techniques [5][6][13][36][37] are those mostly reported for the remaining applications.
Novel FFs have emerged to meet the current demand for more sensitive strategies while ensuring, at the same time, more selectivity. For this purpose, new solvents have been tested as alternative carriers to conventional alcohol-based and water-based FFs. Among these solvents, ILs are highlighted due to their versatile properties, which undoubtedly have contributed to their extensive use in different sample preparation methods. ILs are a group of salts with melting points up to 100 °C characterized by their low to negligible vapor pressure at room temperature. They are formed by bulky and asymmetric organic cations and organic or inorganic anions [45]. Among the interesting features of ILs, their chemical, thermal, and mechanical stability, high solvation ability, together with their tuneability, should be highlighted [46]. Despite the beneficial properties offered by ILs, their synthetic procedures are (in general) non-sustainable. Besides, their high chemical stability makes them non-biodegradable, leading to possible bioaccumulation [47].
Alkylimidazolium ILs have been explored as carriers for the preparation of different IL-based FFs in the last decade. The most common cation of the reported ILs is 1-hexyl-3-methylimidazolium ([C6C1Im]+) in combination with tetrafluoroborate ([BF4]−) [23][24][25] or bis(trifluoromethylsulfonyl) imide ([NTf2]−) [7] anions. Another extensively used cation is 1-butyl-3-methylimidazolium tetrafluoroborate IL ([C4C1Im]+[BF4]−) [21][22][34], whereas Yang et al. reported the application of the IL-based surfactant 1-octyl-3-methylimidazolium hexafluorophosphate ([C8C1Im]+[PF6]−) as a carrier [26]. These ILs have been mixed with a variety of MNPs to form different FFs, with silica-coated Fe3O4-based MNPs being one of the most widely reported [23][24][34], together with Fe3O4@graphene oxide nanospheres [21][22]. Figure 3A) shows scanning electronic microscopy (SEM) images of both neat MNPs and silica-coated MNPs used in these FFs [23].
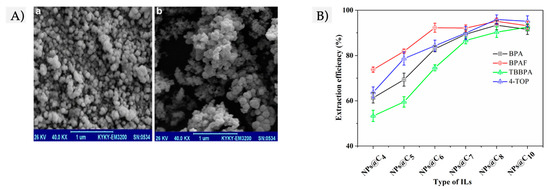
Figure 3. (A) SEM images of (a) Fe3O4-based magnetic nanoparticles (MNPs), and (b) silica-coated Fe3O4-based MNPs, used for the preparation of an ionic liquid (IL)-based FF. Figure adapted from [23] with permission of Springer. (B) Comparison between the extraction efficiencies obtained with different IL-based FFs with the same MNPs but different IL types (modifying the length of the alkyl chain of the IL cation), tested with a group of four endocrine-disrupting phenols. Figure adapted from [26] with permission of Elsevier.
Sodium dodecyl sulfate (SDS) [25] and oleic acid [26] have been incorporated as coatings of Fe3O4-based MNPs of this type of FF with ILs to avoid their agglomeration, despite acetic acid, was also added as a stabilizing agent in most of the applications [21][22][23][24][34]. The study of Zhang et al. used spherical barium ferrite nanoparticles (Ba-αFe MNPs) to prepare IL-based FF [7]. In this case, IL was synthesized separately and then added as an extraction solvent to the sample prior to the addition of MNPs. Thus, the IL-based FF was directly formed in the sample, differing from the rest of IL-based FFs, which are all prepared by mixing the IL with MNPs under sonication before its incorporation into the sample.
Several studies have investigated the tuneability of ILs towards the preparation of FFs with the same MNPs and different IL structures, ultimately intending to establish the influence of the IL on the extraction efficiency of the FFs. Figure 3B) includes a representative graphic of the effect of different types of ILs (that differ on the alkyl chain length) within Fe3O4-based FFs. They were tested by Yang et al. for the determination of phenolic compounds in water and milk samples [26]. The best extraction efficiencies were obtained with the [C8MIm]+[PF6]− IL, and, thus, it was selected as the carrier. It is interesting to mention that the enhanced interactions that were observed between the optimum IL and analytes were accompanied by remarkable stability of the FF. This was due to the large alkyl chain of the cation, that promoted strong hydrophobic interactions between the IL and MNPs while preventing the agglomeration of MNPs. Thus, in this case, the IL has a double role: carrier and stabilizer (mainly, with a steric mechanism, due to the length of the alkyl chain). This is a clear example of how the tuneability of ILs affects the FFs properties and, ultimately, the role that they can play within IL-based FFs, allowing the design of highly specific, stable, and efficient magnetic liquids for sample preparation strategies.
The size of MNPs in the reported IL-based FFs is wide, with diameters between 5 nm [26] and 80 nm [23]. In any case, the maximum amount required in the applications was 50 mg for the MNPs [25], and the required volumes of IL ranged from 50 µL [7] to 300 µL [24]. Thus, these types of IL-based FFs are clearly in the order of microliters.
The extraction step of the methods based on IL-based FFs is mostly instantaneous or only comprises few seconds, taking advantage of the enhanced interaction between analytes and IL as a carrier, and, thus, it does not require any assistance [7][21][23][24][25], with 5 min being the maximum extraction time reported [22][26]. In contrast, sonication is usually required for the desorption of the analytes [7][21][22][23][24][25][34]. It is important to highlight that the selected desorption solvent depends on the specific application. Thus, HCl or HNO3 are used for the determination of metals [21][22][23][24][34], whereas ACN and MeOH are preferred for the remaining applications [7][25][26].
The most popular analytical application of IL-based FFs is shifting toward the extraction of metals [21][22][23][24][34], with methods combined in all the studies with FAAS for their determination. Pesticides [7] and phenols [26] have also been target analytes, using, in these cases, liquid chromatography as the analytical technique. In addition, Ramandi et al. applied an IL-based FF for the extraction of a cationic dye and used UV-Visible spectrophotometry for the analytical determination [25].
The most common samples analyzed in IL-based FFs studies include different environmental [21][22][23][24][25][26][34] and food samples [7][21][23][24][26][34].
Despite all the benefits reported by ILs as carriers for preparing FFs, they face criticism due to their debatable greenness as their synthesis generally requires organic solvents, long refluxing times, and purification steps before their use. In addition, the high chemical stability of ILs, despite being one of their most interesting properties, can be also considered a green drawback as the compounds are not biodegradable, causing a possible accumulation in the environment. In this sense, DES, formed by two main components, a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), arise as a novel alternative of high interest [47].
DESs and ILs have several similarities, such as low vapor pressure, high tunability, non-flammability, and easiness to solvate either organic and inorganic compounds. However, a DES cannot be classified as a type of IL and the terms cannot be used interchangeably, as their nature and preparation differ [48]. The synthesis of DESs can be done by several strategies, but they are mainly prepared by mixing both components (HBA and HBD) under continuous stirring and heating, until a homogeneous liquid is formed. One of the advantages of the preparation of DESs is that there is no need for purification of the product once the synthesis has ended. In addition, a 100% conversion of the precursors to form the desired DES is ensured. Besides, a wide range of different natural products can be used for the synthesis of DESs. For these reasons, they have been suggested as the perfect eco-atomic economical product [49]. Furthermore, natural DESs are less toxic than conventional ILs and they present higher biodegradability [47]. Consequently, all the beneficial properties offered by DESs have been introduced in sample preparation and in analytical chemistry when utilizing these materials as alternative green extractant phases and/or as additives [50][51].
In FFs, DESs have been introduced as potential alternative carriers. Majadi and Hadjmohammadi demonstrated the potential of DESs as carriers in their study using Fe3O4 with and without DES as a carrier [8]. When using the proposed DES-based FF, a synergistic effect between the MNPs and the DES was observed, improving the adsorption capacity and the extraction performance due to the enlargement of the contact surface between the sample solution and the extractant phase [8].
With respect to the use of DESs as carriers for the preparation of FFs, generally, in microextraction approaches, most are binary DESs formed with choline chloride (ChCl) [15][19][20][38], LD-menthol [16][17][39][44], and tetraethylammonium [8] as HBA, and stearic acid [19][20][38], ethylene glycol (EG) [8][15], decanoic acid [39][44], octanoic acid [17], and acetic acid [16], as HBD. A study by Jouyban et al. used a ternary DES based on phosphocholine, menthol, and decanoic acid (1:1:1). This DES showed good analytical performance for the extraction of sixteen PAHs from grilled meat extracts, while assisting the method with air to help increase the contact sample FFs [18].
The proper selection of the HBA and the HBD to form the DES carrier is critical in the resulting properties of the FF [18][19][38][39][44]. As an example, Mohebbi et al. evaluated the use of ChCl:stearic acid, ChCl:palmitic acid, and ChCl:phenylacetic acid, as DES carriers for the extraction of seven pesticides from herbal distillate samples. As shown in Figure 4A, there are noticeable differences in the extraction capacity of the DES-based FF as a function of the HBD used to prepare the DES. The stearic acid as HBD improved the extraction efficiency, as the hydrophobicity of the resulting DES (and, consequently, that of the prepared DES-based FF) was higher. In addition, the viscosity of the DESs-based FF using ChCl:stearic acid DES was lower than for the others, allowing a better dispersion of the extractant material during the extraction and desorption steps [38].
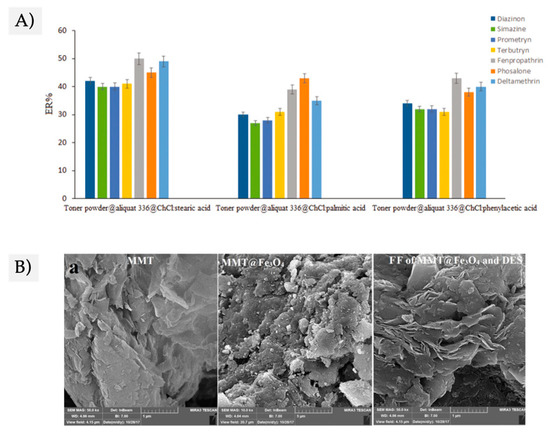
Figure 4. (A) Study of the effect of the hydrogen bond donor (HBD) (selected to prepare the deep eutectic solvents (DES)) on the extraction efficiency of the DES-FF for 7 pesticides. Figure adapted from [38] with permission from Taylor & Francis Group (B) SEM images of neat clay (montmorillonite, labeled as MMT), the clay@Fe3O4 and the clay@Fe3O4: DES, as an example of magnetic nanocomposites rather than other conventional MNPs in FFs. Figure adapted from [44] with permission from Elsevier.
The procedure to prepare DES-based FFs first requires the synthesis of the MNPs, in most cases followed by a further step of functionalization to avoid aggregation [8][15][16][17][18][19][20][38][39][44]. The most common functionalization for MNPs in DES-based FFs includes a silica layer [8][15][18] or the incorporation of the surfactant Aliquat 336 [19][20][38].
Although most of the reported studies use functionalized MNPs, recent reports describe the utilization of magnetic nanocomposites combined with DESs. Zarei et al. reported the preparation of a magnetic clay prepared by coprecipitation of the Fe3O4 in the presence of the montmorillonite, and then combined it with LD-menthol:decanoic acid DES [44]. This study is interesting as it opens a broad field on the use of magnetic nanocomposites in FF. Figure 4B shows SEM images of the clay@Fe3O4: DES.
The amount of DES-based FF used in these applications ranges between 50 µL [44] and 250 µL [15]. Despite these low amounts of DES-based FF, for example, Zarei et al. reported low limits of quantification (lower than 0.91 µg·L−1) and adequate precision (expressed as relative standard deviation) of 8%. In addition, no matrix effect was noticed despite dealing with complex samples, with recoveries in the range of 99.0% to 104% [44].
DES-based FFs have been successfully used in a wide variety of applications such as determination of pesticides [20][37], PAHs [19], flavonoids [8], drugs [15][16][17], metals [39], and explosives [44] among others; in samples such as waters [34][44], soils, [44], body fluids [15][16][17][19], beverages [8][20][28][38], and foods [18]. Despite MDLLME being the main extraction procedure followed using DES-based FFs [8][15][16][17][18][38][39], several authors have proposed other liquid-phase methods with DES-based FFs, such as directly suspended droplet microextraction (DSDME) [44] and air-assisted LPME (AA-LPME) [19]. In DSDME, the FF is placed at the bottom of the vial while stirring at a rate low enough to ensure the maintenance of the drop allowing the easy collection of the FF once the extraction is finished. Although the total recovery of the FF is ensured, longer extraction times are required in this LPME method. On the other hand, the dispersion of the FF in AA-LPME is carried out with cycles of aspiration and ejection using a syringe, making the use of ultrasounds or any additive to disperse the FF unnecessary.
Although reusability is considered a key aspect to consider a material green, this factor has been barely reported for water-based, alcohol-based, IL-based, and DES-based FFs. Nevertheless, the studies performed by Rastbood et al. using a DES-based FF prepared with Fe3O4@SiO2 and ChCl:EG demonstrated a possible reutilization of at least 20 extraction cycles in highly complex biological samples [15]. Besides, Taherimaslak et al. have reported the reutilization of water-based FFs up to 10 times without noticing extraction efficiency [41][42]. Thus, reusability can be expected from these materials. In any case, ongoing studies should focus more attention on this aspect to completely evaluate the potential of FFs as a truly green alternative.
SUPRASs are nanostructured liquids produced in colloidal suspensions of amphiphiles, by a self-assembly and coacervation phenomenon [52][53]. These solvents are characterized by presenting different polarity microenvironments, multiple binding sites, while having the possibility of tailoring their properties. Their preparation is divided into two steps: (i) preparation of a colloidal suspension above the critical aggregation concentration, and (ii) modification of the environmental conditions by introducing a coacervation-inducing agent to increase the size of the aggregates, thus forming droplets with different densities. This leads to the formation of a new liquid phase where the droplets are kept as individual entities [52][54]. Although the greenness and outstanding properties of these solvents, their use is still being not widely extended. To the best of our knowledge, only three studies have been reported on the use of SUPRAS-based FFs [9][28][40]. The main SUPRA used is the one prepared with decanoic acid and tetrabutylammonium salt [9][40]. The previously synthesized MNPs are mixed with the SUPRAS under sonication forming the colloidal suspension of the SUPRA-based FF. The loading charge of nanoparticles used is ~0.5%, and the amount of SUPRAS-based FFs used in the analytical applications reported are between 30 µL [40] and 70 µL [9]. These FFs have been used for the extraction of pesticides in water and fruit juice [28][40] and antibiotics in body fluids [9], showing, in all cases, adequate analytical performance.