1000/1000
Hot
Most Recent

Castration Resistant Prostate Cancer (CRPC) is an advanced stage of Prostate cancer disease. Its evolution is consequent upon androgen withdrawal therapy: which is the first line of therapy for metastatic phenotype. Combination of cytotoxic drugs and hormonal therapy/or genotherapy is a recognized modality for treating CRPC. However, this strategy is limited by poor bio-accessibility and poor efficacy of the cytotoxic drugs. Further increase in dose rather results to an increased rate of collateral toxicity and incidence of multidrug resistance (MDR). Nanovectorization of these strategies has evolved to a promising approach to achieve a more efficacious therapeutic outcomes. It offers the possibility to consolidate their antitumor activity through enhanced specific and less toxic active or passive targeting mechanisms, as well as enabling diagnostic imaging through theranostics. While studies on nanomedicine are common in other cancer types, only a few have focused on prostate cancer. The idea in this article is to reveal possibilities for homig nanomedicine -based formulations into prostate treatment.
Prostate cancer (PC) is the most frequent male cancer in the developed world. The majority of the localized PCs can be treated with surgery or radiation. However, if the disease is diagnosed at the extra-prostatic or metastatic stage, neither radiation nor surgery can offer a good clinical benefit. While Androgen Deprivation Therapy (ADT)/castration represents a consensus treatment for advanced PC, it has become more clear that this disease does not uniformly and completely regress following ADT [1] and may account for the short-lived clinical benefit of 2–3 years [2][3][4]. During this period, most patients become unresponsive to ADT and progress to ADT-resistant PC, a state that is termed Castration-Resistant Prostate Cancer (CRPC) [5][6]. Unfortunately, to date, this phenotype is virtually untreatable and ultimately, patients of this category usually die of the disease. As a sequel to its bad prognosis, CRPC has remained a serious challenge to both clinicians and drug developers.
Given the challenges of collateral toxicity and non-specific distribution of PC therapies, arising from convectional delivery methods, which translates to poor efficacy, scientists have embarked on the search for a veritable alternative in order to contend with these challenges. Nanotechnology provides the platform with inherent characteristics to guarantee the safety, specificity and therapeutic efficacy of advanced prostate cancer therapies. These nanoparticles consist of biodevices and materials with functional ductility and various structural characteristics such as polymers, lipids, inorganic carriers and biological scaffolds to create nanoscale drug carrier systems (nanoparticles) capable of specific delivery of cancer therapeutics [7].
Indeed, with the advent of nanovectors and nanovectorization of PC therapies, it is possible to [1] deliver a high dose of anticancer agents, [2] co-deliver two or more therapeutic molecules in a single nanoformulation, [3] achieve a payload delivery of drug agent, [4]) reduce toxicity and [5] improve therapeutic outcome.
For instance, functionalized nanovectors can consolidate the individual pharmacokinetics and pharmacodynamics of drug agents into one vehicle and increase the likelihood of delivering each agent to the tumor cells at a ratiometric dose [8]. Additionally, we and others have recently demonstrated the possibility to co-deliver a Chemogene (chemo-and-gene based therapy) in a single nanoconstruct to synergize gene silencing and cytotoxicity for CRPC therapy [9][10][11]. Indeed, nanoparticles represent an excellent drug delivery system with enhanced targeted drug delivery capabilities via the passive or the active mechanisms. They have shown to decrease drug toxicity, concentrate drug at disease sites, prolong the systemic circulation of the drug as well as protect drugs from humoral attacks [12]. While prostate cancer therapeutics has not enjoyed sufficient attention in the field of nanomedicine, available data indicate a promising future. For example, near-infrared fluorescence (NIRF) imaging of PC-3 xenograft-bearing mice showed that PEG-micelles were selectively accumulated at the tumor site with minimal distribution in major organs, including liver and spleen [13][14]. Similarly, delivery of paclitaxel via PEG5K-embelin2 micelles leads to superior antitumor activity compared to Taxol in murine models of breast and prostate cancers [14]. Xang and colleagues have reported the impact of oxygenation induced by per-fluoro carbon nanodroplet on accumulation in prostate tumors xenograft. They observed a particle accumulation in mice tumor within 24 h, with a reduction of the tumor hypoxia without enhancing oxygen breathing [15]. With these available testimonies and more on the promises of nanoparticles in CRPC treatment, it is sufficiently acceptable to assert that nanovectorization posits to revolutionize the treatment of CRPC.
Ideally, for a nanovector to be qualified as a drug delivery material, it must be non-toxic, biocompatible, non-immunogenic, biodegradable, possess the ability to avoid the Reticulo-endothelial system (RES) and renal clearance systems [16][17]. These factors are particularly important to ensure that the perceived gains associated with nanovectorization of drugs are effectively maximized.
Currently, nanoparticles are classified according to their chemical compositions (Figure 1): (1) metal-based nanoparticles to include quantum dots, iron oxide and gold nanoparticles, zinc nanoparticle, mesoporous silica, and organic-inorganic nanoparticles [18], (2) carbon-based nanoparticles such as nanotubes or fullerenes [19], (3) polymer nanoparticles such as Nanocapsules or dendrimers [20][21], (4) lipid-based nanoparticles including liposomes and solid lipid nanoparticles [22][23], and (5) a new class based on nucleolipid nanoparticles.
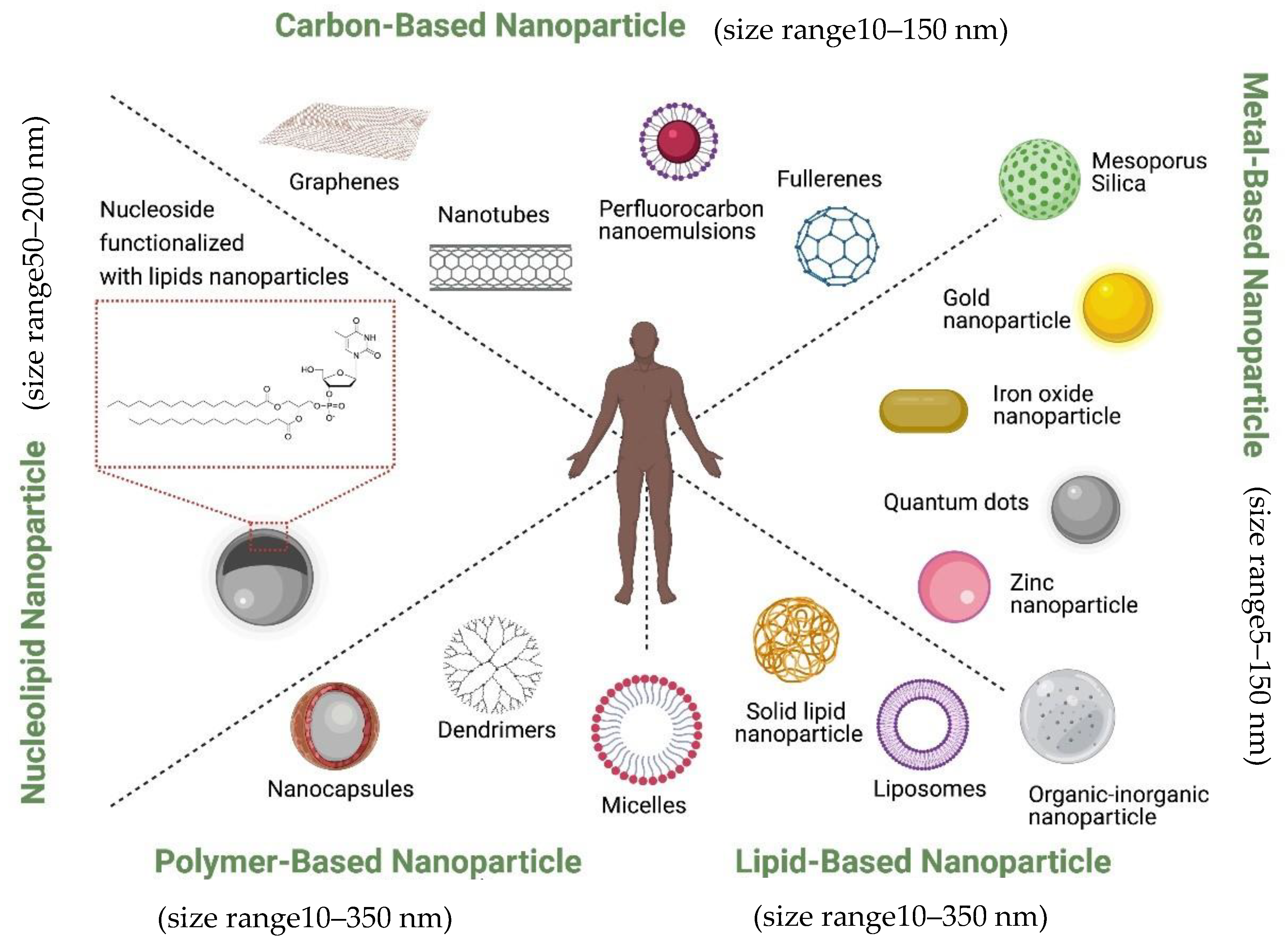
They can also be decorated with functional moieties such as specific ligands to induce active targeting. It is also possible to further innovatively engineer them to bring together the active and passive targeting mechanisms such as, EPR effect, RES avoidance, bio-recognition moieties, membrane trafficking and efficient intracellular delivery, remote drug activation and controlled drug release to act in efficacious operational harmony. Ferrari and colleagues described this group of nanovectors as ‘Logic embedded Vectors’ (LEV) and rationalized their potential in personalized medicine [7].
These nanoparticles are designed to take advantage of the exclusive tumor signatures such as Enhanced Permeability and Retention (EPR) phenomenon, pH, hypoxia, as well as overexpression of tumor-specific receptors [24][25][26] in order to selectively home into tumor cells with minimal/no effect on their normal counterparts.
At this moment, several nanovectorized drugs have received FDA’s approval while some are at different phases of clinical or preclinical development [27][28][29][30]. While the field of nanotherapeutics has been substantially studied, developed and utilized in the treatment of various cancer types, its enormous potentials have been underutilized in prostate cancer therapy, both in preclinical and clinical settings. Here, we review the various conventionally used nanoscale drug carriers such as liposomes, micelles and dendrimer nanovectors. We bring a deep insight into their structural designs and mechanisms of action. Available knowledge on their applications in delivering cancer chemotherapeutics is provided with new specific possibilities in transforming prostate cancer treatment strategies.