1000/1000
Hot
Most Recent

Curcumin (CUR) is a natural substance extracted from turmeric that has antimicrobial properties. Due to its ability to absorb light in the blue spectrum, CUR is also used as a photosensitizer (PS) in antimicrobial Photodynamic Therapy (aPDT).
| Solvent | Microorganism | Culture | Antimicrobial Method | CUR Concentration |
Light/Ultrasonic Parameters | Reference |
|---|---|---|---|---|---|---|
| DMSO (0.4%) | ZIKV | Cell infection | IC50 | 5.62–16.57 µM | - | [19] |
| >DGEV | >IC90 | |||||
| N/R | HPVA | Cell infection | Viral survival | 0.015 mg/mL | - | [20] |
| Tulane V | ||||||
| N/R | KSPV | Infected cells | EC50 | Up to 6.68 µM | - | [21] |
| Aqueous Piper nigrum seed extract | SARS-CoV-2 | Cell infection | IC50 Plaque reduction | 0.4 µg/mL | - | [22] |
| DMSO (<0.4%) | SARS-CoV-1 | Cell infection | Inhibiton of viral replication | 20 µM | - | [23] |
| N/R | SARS-CoV | In vitro | Viral inhibition | 23.5 µM | - | [24] |
| N/R | SARS-CoV | In vitro | papain-like inhibition | 5.7 µM | - | [25] |
| DMSO (1 w/v) | S. aureus | Planktonic | Inhibition zone MIC | 600 and | - | [26] |
| E. coli | 400 µg/mL | |||||
| DMSO | MRSA | Planktonic | MIC FICI |
15.5 µg/mL | - | [27] |
| N/R | S. aureus | Planktonic | Colony count | 100 µg/mL | 8 or 20 J/cm2 | [28] |
| MSSA | ||||||
| MRSA | ||||||
| DMSO (10%) | S. aureus | Biofilm | aPDT | 20, 40, and 80 µM | 5.28 J/cm2 | [29] |
| DMSO | VRSA | Biofilm/animal infection model | MIC MBC |
156.25 µg/mL | 20 J/cm2 | [30] |
| N/R | S. aureus | Animal infection model | aPDT | 78 µg/mL | 60 J/cm2 | [31] |
| DMSO | S. aureus | Infected fruit | Survival fraction | 100 nM | 1.5 and 9 J/cm2 | [32] |
| N/R | S. aureus | Planktonic | PDI | 40 and 80 µM | 15 J/cm2 | [33] |
| E. coli | ||||||
| Tween 80 (0.5%) | S. aureus | Planktonic | CFU/mL | 300 and 500 µM | 0.03–0.05 W/cm2 | [34] |
| N/R | S. aureus | Biofilm | Confocal microscope | N/R | 170 µmol m2 s1 | [35] |
| DMSO (0.5%) | S. aureus | Biofilm | SDT aPDT SPDT |
80 µM | 100 Hz 15 and 70 J/cm2 100 Hz, 15 and 70 J/cm2 |
[36] |
| DMSO | E. coli | Planktonic | MIC Inhibition zone |
110, 220 and 330 µg/mL | - | [37] |
| DMSO | E. coli | Planktonic | OD600nm | 8,16, 32, and 64 µg/mL | - | [38] |
| N/R | S. dysenteriae | Planktonic | MIC/MBC | 256 and | - | [39] |
| C. jejuni | 512 µg/mL | |||||
| Edible alcohol | E. coli | Planktonic | aPDT | 5, 10, and 20 µM | 3.6 J/cm2 | [40] |
| DMSO | H. pylori | Planktonic biofilm | MIC MBC aPDT |
50 µg/mL | 10 mW/cm2 | [41] |
| DMSO | P. aeruginosa | Biofilm | aPDT CFU/mL |
N/R | 5 and 10 J/cm2 | [42] |
| DMSO | Imipenem-resistant A. baumannii |
Planktonic | aPDT | 25, 50, 100, and 200 µM | 5.4 J/cm2 | [43] |
| DMSO (2%) | P. aeruginosa, A. baumannii, K. pneumoniae, E. coli, E. faecalis | Planktonic | MIC/FICI | 128-256 µg/mL | - | [44] |
| N/R | C. difficile, C. sticklandii, B. fragilis, P. bryantii | Planktonic | Viable cell number | 10 µg/mL | - | [45] |
| N/R | B. subtillis, E. coli, S. carnosus, M. smegmatis | Planktonic | MIC/MBC | Up to 25 µM | - | [46] |
| N/R | MRSA | Planktonic/animal infection model | MIC | 4–16 μg/mL | - | [47] |
| MSSA | 2–8 μg/mL | |||||
| E. coli | 8–32 μg/mL | |||||
| N/R | E. faecalis, S. aureus, B. subtillis, P. aeruginosa, E. coli | Planktonic | MIC | 156 μg/mL | - | [48] |
| DMSO (0.5%) | A. hydrophila, E. coli E. faecalis, K. pneumoniae, P. aeruginosa, S. aureus, C. albicans |
Planktonic | MIC/MBC/ FICI/aPDT |
37.5–150 µg/mL | N/C | [49] |
| N/R | E. faecalis | Infection model | CFU/mL | 1 µg/mL | - | [50] |
| Commercial solution | E. faecalis | Biofilm | aPDT | 1.5 g/mL | 20.1 J/cm2 | [51] |
| Ethanol 99% | A. hydrophila, V. parahaemolyticus | Planktonic | aPDT/SDT | Up to 15 mg/L | N/C | [52] |
| DMSO (10%) | E. faecalis | Biofilm | MIC/MBC | 120 mg/mL | - | [53] |
| N/R | S. mutans | Planktonic | aPDT | 10 g/100cc | N/C | [54] |
| DMSO: ethyl alcohol | S. mutans, S. pyogenes | Planktonic | aPDT | 3 mg/mL | 28.8 J/cm2 | [55] |
| DMSO (0.8%) | Caries isolated | Biofilm | aPDT | 600 µg/mL | 75 J/cm2 | [56] |
| DMSO | S. mutans, C. albicans | Biofilm single/dual | MBEC | 0.5 mM | - | [57] |
| DMSO (0.05 M) | A. actinomycetemcomitans | Planktonic | aPDT | 40 µg/mL | 300–420 J/cm2 | [58] |
| DMSO (<1%) | P. gingivalis, A. actinomycetemcomitans | Planktonic | aPDT | 20 µg/mL | 6, 12 or 18 J/cm2 | [59] |
| DMSO (0.5%) | P. gingivalis, A. actinomycetemcomitans, C. rectus, E. corrodens, F. nucleatum, P. intermedia, P. micra, T. denticola, T. forsythis | Biofilm | aPDT | 100 mg/L | - | [60] |
| N/R | Subgingival plaque | Biofilm | aPDT | 100 µg/mL | 30 J/cm2 | [61] |
| DMSO | P. gingivalis | Planktonic | MIC | 12.5 µg/mL | - | [62] |
| Ethanol: DMSO (99.9%: 0.1%) |
Periodontal pocket | - | aPDT | 100 mg/mL | 7.69 J/cm2 | [63] |
| Tween 80 | Streptococcus spp, Staphylococcus spp, Enterobacteriaceae, C. albicans | Clinical trail | aPDT | 0.75 mg/mL | 20.1 J/cm2 | [64] |
| Sodium hydroxide: PBS | C. albicans, C. parapsilosis, C. glabrata, C.dubliniensis | Planktonic/biofilm | MIC | 0.1–0.5 mg/mL | - | [65] |
| N/R | C. albicans, S. aureus | Planktonic Biofilm |
MIC/Biofilm percentag | 200 µg/mL | - | [66] |
| N/R | C. albicans | Biofilm | aPDT | 1.5 g/mL | 20.1 J/cm2 | [67] |
| DMSO (10%) | C. albicans | Biofilm | aPDT | 20, 40, 60 and 80 µM | 2.64, 5.28, 7.92, 10.56, and 13.2 J/cm2 | [68] |
| DMSO (1%) | C. albicans | Biofilm | aPDT | 40 and 80 mM | 37.5 and 50 J/cm2 | [69] |
| N/R | C. albicans | Biofilm | aPDT | 100 µM | 10 J/cm2 | [70] |
| DMSO (2.5%) | Fluconazole-resistant C. albicans | Planktonic/biofilm/infection model | MIC/ aPDT |
40 µM | 5.28 J/cm2 | [71] |
| Fluconazole-susceptible C. albicans | 80 µM | 40.3 J/cm2 | ||||
| DMSO | C. albicans, F. oxysporum, A. flavus, A. niger, C. neoformans | Planktonic | MIC | 137.5–200 μg/mL | - | [72] |
The antifungal activity of CUR has been demonstrated mostly against Candida spp. by many in vitro and few in vivo studies [90]. CUR inhibited the growth of a reference strainand a clinical isolate of C. albicans, as well as reference strains of Candida parapsilosis, Candida glabrata, and Candida dubliniensis [65]. When biofilms of both C. albicans strains were evaluated, CUR reduced only the viability of the standard strain in a concentration-dependent effect, while the antifungal fluconazole did not inhibit the viability of either strain [65]. CUR and 2-aminobenzimidazole (2-ABI) inhibited the growth and adhesion of C. albicans and S. aureus to medical-grade silicone [66]. The combination of CUR and 2-ABI enhanced the inhibition of biofilm formation and reduced the viability of 48 h-old single and dual-species biofilms [66]. The aPDT mediated by CUR reduced the survival of 14-day-old biofilm of C. albicans in bone cavities, confirmed by fluorescence spectroscopy [67]. CUR-mediated aPDT reduced the metabolic activity of biofilms of C. albicans reference strain and clinical isolates from the oral cavity of patients with HIV and lichen planus [68]. Moreover, genes related to hyphae and biofilm formation were downregulated [68]. The aPDT mediatedby CUR and another PS, Photodithazine®, also resulted in the downregulation of genes involved in adhesion and oxidative stress response in C. albicans biofilms [69]. CUR alone and CUR-mediated aPDT, combined or not with an antibody-derived killer decapeptide,reduced the metabolic activity of an 18 h biofilm of C. albicans [70]. CUR showed synergism with fluconazole and CUR-mediated aPDT inhibited the planktonic growth and reduced the biofilm viability of fluconazole-resistant C. albicans [71]. CUR-mediated aPDT also increased the survival of Galleria mellonella infected with fluconazole-susceptible C. albicans, but did not affect the survival of larvae infected with fluconazole-resistant strain [71]. A library of 2-chloroquinoline incorporated monocarbonyl curcuminoids (MACs) was synthetized and most of the MACs exhibited strong or moderate antifungal activity compared with miconazole against C. albicans, Fusarium oxysporum, Aspergillus flavus, Aspergillus niger, and Cryptococcus neoformans [72]. To suggest a possible antifungal mechanism, a moleculardocking analysis showed that MACs had binding affinity to sterol 14α-demethylase(CYP51), leading to impaired fungal growth [72].
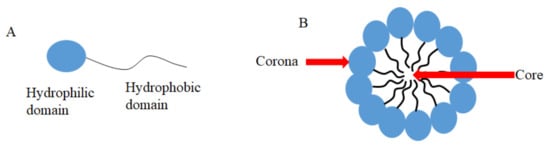
| Type of Micelles | [CUR] Formulation | Microorganism | Type of Culture |
Antimicrobial Method | Antimicrobial [CUR] | Light/Ultrasonic Parameters | Reference |
|---|---|---|---|---|---|---|---|
| Mixed polymer micelles | 1000 ppm | E. coli, S. aureus, A. niger | Planktonic | MIC | 350 and 275 µg/mL | - | [92] |
| PCL-b-PAsp and Ag | 2 mg/mL | P. aeruginosa, S. aureus | Planktonic | OD600nm | 8–500 µg/mL | - | [93] |
| mPEG-OA | 1:10 | P. aeruginosa | Planktonic | MIC | 400 µg/mL | - | [94] |
| PEG-PCL | 10 mg | C. albicans | Planktonic | MIC | 256 µg/mL | - | [95] |
| PEG-PE | 50 mM | S. mutans | Planktonic | SACT | 50 mM | 1.56 W/cm2 | [96] |
| DAPMA, SPD, SPM | 0.32 mg/mL | P. aeruginosa | Planktonic | OD600nm and aPDT | 250, 500 nM, 1 µM and 50, 100 nM | 18 and 30 J/cm2 | [97] |
| P123 | 0.5% w/V | S. aureus | Planktonic | aPDT | 7.80 μmol/L | 6.5 J/cm2 | [98] |
| PCL-b-PHMG-b-PCL, STES | 10 mg | S. aureus,E. coli | Planktonic | MIC | 16 and 32 μg/mL * | - | [99] |
| CUR-PLGA-DEX | 1 mg/mL | P. fluorescens, P. putida | Planktonic biofilm | OD600nm antibiofilm | 0.625–5 mg/mL | - | [100] |
Liposomes are biodegradable and biocompatible systems, which consist of hydrophobic and hydrophilic groups (Figure 2) [101]. The hydrophobic layer is mainly composed of phospholipids and cholesterol molecules. This lipid-based carrier is suitable for administering water-insoluble drugs, such as CUR [102]. Liposomes are classified into three groups: single unilamellar vesicles, large unilamellar vesicles, and multilamellar vesicles [103]. Drugs encapsulated in liposomes are protected from chemical degradation and show increased drug solubility [101]. Additionally, liposomes have advantageous properties such as better penetration into the skin, deposition, anti-melanoma, and antimicrobial activity [102]. Antimicrobial studies with CUR-loaded liposomes are summarized in Table 3.
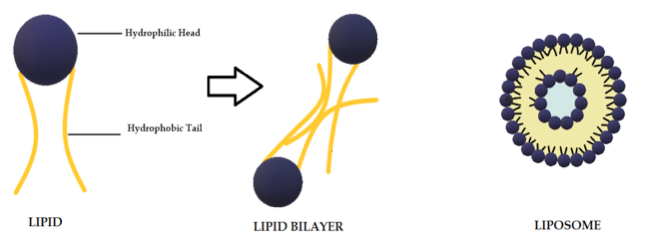 Figure 2. Schematic representation of the liposome structure.
Figure 2. Schematic representation of the liposome structure.
Table 3. Antimicrobial studies performed with CUR in liposomes and solid lipid nanoparticles (SLN).
| Type of Liposomes or SLN | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Reference |
| Lecithin and cholesterol | 0.5 mg/mL | A. sobria, C. violaceum, A. tumefaciens | Planktonic biofilm | MIC, antibiofilm | 420, 400, and 460 μg/mL | [104] |
| PCNL | 60.65 ± 1.68 µg/µl | B. subtilis, K. pneumoniae, C. violaceum, E. coli, M. smegmatis, A. niger, C. albicans, F. oxysporum | Planktonic | Disk diffusion assay | N/R | [105] |
| Phosphocolines | 100:1 M | S. aureus | Planktonic | MIC | 7 μg/mL | [106] |
| PLGA: triglycerides: F68 | 0.8 mg/mL | E. coli, S. typhimurium, P. aeruginosa, S. aureus, B. sonorensis, B. licheniformis | Planktonic | MIC | 75 and 100 μg/mL | [107] |
| Soya lecithin and menthol | 0.5 mg/mL | MRSA | Planktonic, Biolfim | MIC, microscopy, biomass | 10 and 125 µg/mL | [108] |
| CurSLN | 60 mg/500 mg lipid | S. aureus, S. mutans, V. streptococci, L. acidophilus, E. coli, C. albicans | Planktonic | MIC, MBC | 0.09375–3 and 1.5–6 mg/mL | [109] |
[CUR]: CUR concentration. N/R: not reported.
Solid lipid nanoparticles (SLN, Figure 3, Table 3) are a modern type of lipid-based carrier composed by solid biodegradable lipids and spherical solid lipid particles. SLNs are water colloidal or aqueous surfactant solution systems [102]. SLNs have advantages such as biocompatibility, biodegradability, greater drug absorption, and drug retention [18][102], thus they are an interesting system to carry CUR [14]. Currently, SLNs have become popular because they are used as carriers for COVID-19 vaccines based on RNA vaccine technology (Moderna and Pfizer–BioNTech).
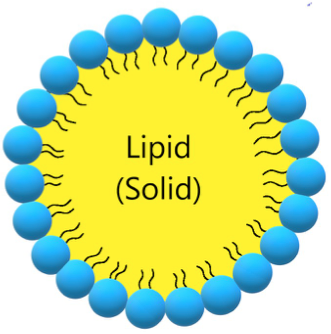 Figure 3. Schematic representation of solid lipid nanoparticle.
Figure 3. Schematic representation of solid lipid nanoparticle.
Nanoemulsions (NE) are thermodynamically stable dispersions of oil and water (Figure 4) [110]. They are formed by a phospholipid monolayer composed of a surfactant and co-surfactant, which are important for nanoemulsion stabilization [110][111]. This system has thermodynamic stability and high solubilization characteristics, with improved drug release kinetics [112]. NE systems can be manufactured through emulsification, which can control the size of the drops and increase the drug solubility and efficacy. Moreover, the main disadvantage of NE is the high amount of surfactants in the formulation, which can lead to a potential toxic effect [111]. Antimicrobials studies with CUR-loaded NE are summarized in Table 4.
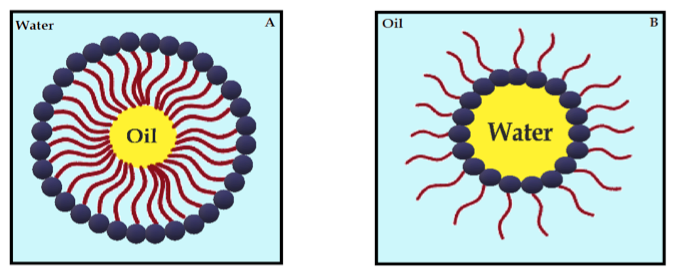 Figure 4. Schematic diagram of oil-in-water nanoemulsion (A) and water-in-oil nanoemulsion (B), stabilized by surfactants.
Figure 4. Schematic diagram of oil-in-water nanoemulsion (A) and water-in-oil nanoemulsion (B), stabilized by surfactants.
Table 4. Antimicrobial studies performed with curcumin/curcuminoid in emulsions.
Type ofEmulsion |
[CUR] Formulation | Microorganisms | Type of culture | Antimicrobial method | Antimicrobial Concentration | Light/Ultrasonic Paramet | Reference |
| THC ME | 5% | HIV-1 | Cell infection | IC50 | 0.9357 μM | - | [113] |
| CUR-NE | N/R | HPV | - | aPDT | 80 µM | 50 J/cm2 | [114] |
| CUR-NE | N/R | DENV-1 to 4 | Cell infection | Cell viability | 1, 5, 10 µg/mL | - | [115] |
| P60-CUR | 4 mg/L | E. coli | Planktonic | OD595 nm | N/R | - | [116] |
| PE:CUR | 0.566 mg/mL | S. aureus, S. epidermidis, S. faecalis, C. albicans, E. coli | Planktonic | Inhibition zone | 1 mg/mL* | - | [117] |
| cu-SEDDS | 1% | E. aureus, E. coli, P. aeruginosa, K. pneumonia | Planktonic | MIC | 45–62 µg/mL | - | [118] |
| CUR:NE in microbeads | 0.5 mg/mL | E. coli, S. typhmerium,Y. enterocolitica, P. aeruginosa, S, aureus, B. cereus, L. monocytogenes | Planktonic | Inhibition zone | 90 and 180 mg/mL* | - | [119] |
| Lignin sulfomethylated | 0.3 mg/mL | S. aureus | Planktonic | OD600 nm | 2.4 mg/mL* | - | [120] |
| C14-EDA/GM/WC14-MEDA/GM/W | N/R | C. albicans | Planktonic, biofilm | Microdilution assay, antibiofilm | 100 µg/mL, 20 µg | - | [121] |
[CUR]: CUR concentration. -: not performed. N/R: not reported. *: formulation concentration.
Cyclodextrins (CDs) have revolutionized the pharmaceutical industry in recent years [122]. CDs consist of three naturally occurring oligosaccharides in a cyclic structure produced from starch [123][124][125]. The natural CDs have their nomenclature system and their chemical structure based on the number of glucose residues in their structure: 6, 7, or 8 glucose units, which are denominated α-CD, β-CD, and γ-CD, respectively [126][127]. Although the entire CD molecule is soluble in water, the interior is relatively non-polar and creates a hydrophobic microenvironment. Therefore, CDs are cup-shaped, hollow structures with an outer hydrophilic layer and an internal hydrophobic cavity (Figure 5) [126]. They can sequester insoluble compounds within their hydrophobic cavity, resulting in better solubility and consequently better chemical and enzymatic stability [124]. Due to the cavity size, β-CD forms appropriate inclusion complexes with molecules with aromatic rings [128], such as CUR [129]. Antimicrobial studies with CUR in CDs are summarized in Table 5.
 Figure 5. Schematic representation of CUR in CD.
Figure 5. Schematic representation of CUR in CD.
Table 5. Antimicrobial studies performed with CUR in CDs.
| Type of CD | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Light/Ultrasonic Parameters | Reference |
| PEG-based β-CD or γ-CD | 10 µM | E. coli, E. faecalis | Planktonic | aPDT | 10 µM | 4.8, 29 J/cm2 | [130] |
| HPMC-stabilized hydroxypro pyl-β-CD | 7.64 × 10−3 M | E. coli | Planktonic | aPDT | 10, 25 µM | 5, 14, 28 J/cm2 | [131] |
| methyl-β-CD hyaluronic acid HPMC | 7.64 × 10−3 M | E. faecalis, E. coli | Planktonic | aPDT | 0.5–25 µM | 11, 16, 32 J/cm2 | [132] |
| carboxymethyl-β-CD | 20 µM | E. coli | Planktonic | aPDT | 0.7 ± 0.1 to 4.1 ± 1.6 nmole cm−2 | 1050 ± 250 lx | [133] |
| hydrogel with CUR in hydroxypropyl-β-CD | 15.8 mg/mL | S. aureus | Planktonic | Inhibition zone | 2% (w/v) | - | [134] |
| α- and β-CD | 1 mol/L | E. coli, S. aureus | Planktonic | MIC, OD600 nm | 0.25 and 0.31 mg/mL | - | [135] |
| β-CD or γ-CD in CS | 0.06 mM | E. coli, S. aureus | Planktonic | MIC, Zone of inhibition | 64 and 32 µg/mL | - | [136] |
| γ-CD | 25 mg/L | T. rubrum | Planktonic | MIC, aPDT | N/R | 45 J/cm2 | [137] |
| hydroxypropyl-β-CD | 1:1 | B. subtillis, S. aureus, S. pyrogenes, P. aeruginosa, C. difficile, C. butyricum, L. monocytogenes, E. faecalis, E. coli, K. pneumoniae, P. mirabilis, S. typhimurium, E. aerogens, C. kusei, C. albicans | Planktonic | Inhibition zone | 25 mg/mL | - | [138] |
| methyl-β-CD | 20 mM | E. coli | Planktonic | MIC, MBC, aPDT | 500, 90 µM | 9 J/cm2 | [139] |
[CUR]: CUR concentration. -: not performed. N/R: not reported.
Chitin is a natural polysaccharide commonly found in the exoskeleton of marine crustaceans such as shrimps, prawns, lobsters, and crabs. Chitosan (CS) derives from the acetylation of chitin and has a linear structure of D-glucosamine (deacetylated monomer) linked to N-acetyl-D-glucosamine (acetylated monomer) through β-1,4 bonds [140]. The main advantages that make CS a promising drug carrier include biocompatibility, biodegradability, non-toxicity, controlled release system, mucoadhesive properties, and low cost [140][141]. Moreover, CS is soluble in aqueous solutions and is the only pseudo-natural polymer with a positive charge (cationic) [142], which can interact with negatively-charged DNA, membranes of microbial cells, and biofilm matrix [143]. Antimicrobial studies with CUR in CS are summarized in Table 6.
Table 6. Antimicrobial studies performed with CUR in CS.
| Type ofCS | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Reference |
| PEG-CS | 4.4%, 5 mg/mL | MRSA, P. aeruginosa | Planktonic, Animal model | OD600nm, CFU | 5 and 10 mg/mL * | [144] |
| CCS microspheres | 12.27 mg/mL, 1 mol | S. aureus, E. coli | Planktonic | Zone of inhibition, MIC | N/R | [145] |
| CS nanoparticles | 1.06 mg/mL | S. mutans | Planktonic, Biofilm | MIC | 0.114 mg/mL | [146] |
| CS-CMS-MMT | 0.0004–0.004 g | S. mutans | Planktonic, Biofilm | MIC | 0.101 mg/mL | [147] |
| CS-GP-CUR | 148.09 ± 5.01 µg | S. aureus | Planktonic | Zone of inhibition, tissue bacteria count | N/C | [148] |
| PVA-CS-CUR | N/C | E. coli, P. aeruginosa, S. aureus, B. subtilis | Planktonic | Zone of inhibition | N/R | [149] |
| PVA-CS-CUR | 10, 20, 30 mg | P. multocida, S. aureus, E. coli, B. subtilis | Planktonic | Zone of inhibition | 10, 20, 30 mg | [150] |
| CS NPs | 2, 4, 8, 16% | C. albicans, S. aureus | Planktonic, Biofilm | MIC, Colony count | 400 mg/mL | [151] |
| CS NPs | 4 mg/mL | HCV-4 | N/R | Antiviral assay | 15 µg/mL | [152] |
| CS/milk protein nanocomposites | 100 mg | PVY | Plant infection | Antiviral activity | 500, 1000, 1500 mg/100 mL | [153] |
Antimicrobial studies with CUR loaded in other polymeric DDSs are summarized in Table 7.
Table 7. Antimicrobial studies performed with curcumin in polymeric drug delivery systems.
| Type of Polymeric DDS | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Light/Ultrasonic Parameters | Reference |
| PEG 400γ-CD and PEG + β-CD | 0.18% | E. faecalis, E. coli | Planktonic | CFU/mL aPDT | N/R | 9.7 J/cm2 29 J/cm2 | [154] |
| CUR-NP without polymer | 100 mg | S. aureus, B. subtillis, E. coli, P. aeruginosa, P. notatum, A. niger | Planktonic | MIC Inhibition zone | 100 mg, 0.27 mmol | - | [155] |
| CUR-NP without polymer | 100 mg | M. lutues, S. aureus, E. coli, P. aeruginosa | Planktonic | MBC | N/R | - | [156] |
| Mixed polymer NP | 5 mM | E. coli | Planktonic | MIC | 400–500 μM | - | [157] |
| CTABTween 20Sodium dodecylsulfate | 100 mg/mL | L. monicytogenes | Planktonic | Inhibition zone | N/R | - | [158] |
| PLA/dextran sulfate | 4 mg/mL | MRSA, C. albicans, S. mutans | Planktonic/mono- and –mixed biofilm | aPDT | 260 μM | 43.2 J/cm2 | [159] |
| PLA/dextran sulfate | 0.4% | C. albicans | Animal model | aPDT | 260 μM | 37.5 J/cm2 | [160] |
| Nanocurcumin | N/R | P. aeruginosa (isolates) and standard strain | Planktonic | MIC | 128 µg/mL | - | [161] |
| PLGA | 5 mg | S. saprophyticus subsp. Bovis, E. coli | Planktonic | aPDT | 50 µg/mL | 13.2 J/cm2 | [162] |
| Eudragit L-100 | N/C | L. monocytogenes | Planktonic | Animal model infection | N/R | - | [163] |
| nCUR | N/R | S. mutans | PlanktonicBiofilm | Inhibition zoneaPDT | N/R | 300–420 J/cm2 | [164] |
| nCUR combined with indocyanine | 100 mg | E. faecalis | Biofilm | Metabolic activity | N/R | 500 mW/cm2 | [165] |
| PVAc-CUR-PET-PVDC | 0.02 g | S. aureus, S. tiphimurium | Planktonic | aPDT | N/R | 24, 48, and 72 J/cm2 | [166] |
| MOA.CUR-PLGA-NP | Up to 10% | S. mutans | Biofilm | aPDT | 7% wt | 45 J/cm2 | [167] |
| CS- β-CD | N/C | S. aureus, E. coli | Planktonic | Colony count | Up to 0.03% | - | [168] |
Metal complexation plays an important role in the therapeutic properties of CUR. The β-diketone moiety in the CUR chemical structure enables it to form complexes with metal ions [169]. A previous review summarized the antimicrobial activity of CUR and curcuminoid complexes with metals, such as boron, Ca2+, Cd2+, Cr3+, Co2+, Cu2+, Fe3+, Ga3+, Hg2+, In3+, Mn2+, Ni2+, Pd2+, Sn2+, Y3+, and Zn2+ against viruses, bacteria, and fungi [169]. Metals have also been combined with polymers to improve the biological effects of CUR and to be used as films, hydrogels, dressings, and other pharmaceutical formulations [170][171]. In this context, silver NPs (AgNPs) have been extensively used due to their antimicrobial activity (Figure 6) [172]. Antimicrobial studies with CUR complexes with metals are summarized in Table 8.
 Figure 6. Schematic representation of CUR in silver nanoparticles.
Figure 6. Schematic representation of CUR in silver nanoparticles.
Table 8. Antimicrobial studies performed with CUR complexes with metallic NPs.
| Type of Metallic Material | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Reference |
| CUR-AgNPs | 20 mg/mL | P. aeruginosa, E. coli, B. subtilis, S. aureus | Planktonic | MIC | 20 mg/mL | [173] |
| Ag-CUR-nanoconjugates | 0.1 mM | E. coli, Salmonella spp., Fusarium spp., S. aureus | Planktonic | Zone of Inhibition | 0.1 mM | [174] |
| AgCURNPs | 500 mg | P. aeruginosaS. aureus | Biofilm | CLSM SEM | Up to 400 μg/mL | [175] |
| AgNPs | 7 mg | E. coli | Planktonic | Turbidimetric Assay | 0.005 µM | [176] |
| cAgNPs | 7 mg | E. coliB. subtilis | Planktonic | MIC, CFU/mL | 7 mg | [177] |
| Ru II complex | 0.092 g | E. coli, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterococcus sp. | Plakntonic | MIC/FICI | >64 µg/mL | [178] |
| SCMC SNCF nanocomposites with CUR | 0.25 mg/mL | E. coli | Planktonic | Disc Method Count Method | 2 mg/mL | [179] |
| CSCL CUR-AgNP | 0.092 g | E. coli, B. subtilis | Planktonic | Zone of Inhibition | 10 and 20 μM | [180] |
| nSnH | 10% | S. aureus, E. coli. | Planktonic | CFU/mL | N/R | [181] |
| Nanocomposite of CUR and ZnO NPs | N/C | S. epidermidis, S. hemolyticus, S. saprophyticus | Planktonic | Zone of Inhibition | 1000, 750, 500, 250 μg/mL | [182] |
| Thermo-responsive hydrogels | N/C | S. aureusP. aeruginosaE. coli | Planktonic | MIC | 400 μg/mL | [183] |
| CUR-AgNPs | 5 mg/mL | C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei, C. kefyr | Planktonic | Zone of Inhibition, MIC | 32.2–250 μg/mL | [184] |
| Gel-CUR-Ag | 20 mg | P. aeruginosa, S. aureus | Planktonic | MIC, MBC | 20 mg | [185] |
| HGZ-CUR | N/C | S. aureus, T. rubrum | Planktonic | Zone of Inhibition | N/C | [186] |
| CHG-ZnO-CUR | N/C | S. aureus, T. rubrum | Planktonic | Zone of Inhibition | N/C | [187] |
| Copper (II) oxide NPs | 1 g | E. faecalis, P. aeruginosa | Planktonic | Zone of Inhibition CFU/mL | 1 mg/mL | [188] |
| OA-Ag-C | 1 g | P. aeruginosa, S. aureus | Planktonic | OD600nm | 2.5 mg/mL | [189] |
| Ag-NP-β-CD-BC | 0.79 g | P. aeruginosa, S. aureus, C. auris | Planktonic | Zone of Inhibition | N/R | [190] |
| Cotton fabrics coated ZnO-NP | 2.71 × 10−3 M | S. aureus, E. coli | Planktonic | Bacterial Count | N/R | [191] |
| CS-ZnO-CUR | 0.2 g | S. aureus, E. coli | Planktonic | MIC, MBC | Up to 50 μg/mL | [192] |
| CUR-TiO2 -CS | 100–300 mg | S. aureus, E. coli | Planktonic Animal infection | MIC | 10 mg | [193] |
| CUR-Au-NPs | 1 mg/mL | E. coli, B. subtilis, S. aureus, P. aeruginosa | Planktonic | Zone of Inhibition | 100, 200, 300 μg/mL | [194] |
Porous materials are structures with ordered pores ranging from nanometer to micrometers, which are classified as microporous (less 2 nm), mesoporous (from 2 up to 50 nm), and macroporous (above 50 nm) [195]. Porous materials can be synthesized using carbon, silica, and metal oxides [196]. Mesoporous silica nanoparticles (MSN, Figure 7) are inorganic scaffolds [197], which seemed ideal carriers for hydrophobic drugs due to their well-defined structure, large specific surface area, and versatile chemistry for functionalization [198]. The pore size and volume and the surface area, as well as the surface functionalization of the mesoporous material, determine the drug load and release [199]. Moreover, mesoporous materials can be modified or functionalized to control drug release under environmental stimuli, such as pH, temperature, or light. These stimuli-responsive DDS, or smart DDS, prevent undesirable drug release before reaching the target tissue (“zero premature release”) [199]. Antimicrobial studies with CUR in porous DDSs are summarized in Table 9.
 Figure 7. Schematic representation of a porous particle.
Figure 7. Schematic representation of a porous particle.
Table 9. Antimicrobial studies performed with CUR in porous DDSs.
| Porous DDS | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Light/Ultrasonic Parameters | Reference |
| Cu-SNP/Ag | 1.0 mmol | E. coli | Planktonic | aPDT | N/R | 72 J/cm2 | [200] |
| Bionanocomposite silica/chitosan | 100 mg | E. coli, S. aureus | Planktonic | Zone of inhibition | N/R | - | [201] |
| NCIP | 1 mg | HIV-1 | Transfected cells | Immuno fluorescent staining | 5–8 mg/mL | - | [202] |
| Lollipop-like MSN | 30 mg L−1 | E. coli, S. aureus | Planktonic | OD600nm | N/R | - | [203] |
| SBA-15/PDA/Ag | 2 mg | E. coli, S. aureus | Planktonic | CFU/mL | 50 mM | - | [204] |
Quantum dots (QDs) are semiconductor particles at nanosize (up to 10 nm) with electrical and photoluminescence properties of biotechnological and biomedical applications, such as bioimaging and DDS [205]. Carbon dots are divided into carbon QD and graphene QD and are produced by top-down and bottom-up methods using bulk carbon material and molecular precursors, respectively [205]. Antimicrobial studies with CUR in QDs are summarized in Table 10.
Table 10. Antimicrobial studies performed with CUR in quantum dots (QDs).
| Type of Material |
[CUR] Formulation |
Microorganism | Type of Culture |
Antimicrobial Method |
Antimicrobial [CUR] |
Light/Ultrasonic Parameters |
Reference |
| CUR-cQDs | 0.6 | S. aureus MRSA E. faecalis E. coli K. pneumoniae P. aeruginosa |
Planktonic Biofilm |
Grown inhibition Biomass evaluation Confocal microscopy |
3.91– 7.825 µg/mL |
- | [206] |
| CUR-cQDs | 200 mg | EV-71 | Cell infection Animal infection |
MIC Plaque assay TC IC50 assay Western blot PCR |
5 µg/mL | - | [207] |
| CUR-MQD | 2:1 wt% | K. pneumoniae P. aeruginosa S. aureus |
Planktonic | MIC MBC Confocal microscopy Fluorescence microscopy Flow cytometry |
<0.00625– 0.125 µg/mL |
- | [208] |
| CUR-GQDs | N/C | A. actinomycetemcomitans P. gingivalis P. intermedia |
Mixed biofilm | aPDT | 100 µg/mL | 60–80 J/cm2 | [209] |
Antimicrobial studies with CUR in films, hydrogels, and other nanomaterials are summarized in Table 11.
Table 11. Antimicrobial studies performed with CUR in films, hydrogels, and other nanomaterials.
| Type of Material | [CUR] Formulation | Microorganism | Type of Culture | Antimicrobial Method | Antimicrobial [CUR] | Light/Ultrasonic Parameters | Reference |
| CuR-SiNPs | 20 mg | S. aureus, P. aeruginosa | Planktonic, Biofilm | aPDT | 50 μg/mL, 1 mg/mL | 20 J/cm2 | [210] |
| CUR-HNT-DX | 10 mg | S. marcescens, E. coli | Planktonic, Infection model | Grown inhibition, Confocal microscopy | Up to 0.5 mg/mL | - | [211] |
| Exosomes | N/R | HIV-1 infection | - | Flow cytometry | N/R | - | [212] |
| Electrospun nanofibers | 100 mg/mL | Actinomyces naeslundii | Biofilm | aPDT | 2.5 and 5 mg/mL | 1200 mW/cm2 | [213] |
| Ga NFCD-GO NF | 0.1 mol | B. cereus, E. coli | Planktonic | Zone of inhibition, MIC | Up to 63.25 µg/mL | - | [214] |
| Multinanofibers-film | 1, 2.5, and 5 mg/mL | S. aureusE. coli | Planktonic | UFC/mL, Confocal microscopy | 1 mg/mL | - | [215] |
| Nanofibers scaffolds | 4.0 wt% | S. aureus Pseudomonassp. | Planktonic | Colony count | N/R | ,- | [216] |
| Nanofibrous scaffold | 5% | S. aureus, E. coli | Planktonic | Colony count | 20 mg | - | [217] |
| Nanofibers | 5 and 10%wt | S. aureusE. coli | Planktonic | OD600nm | Up to 212.5 µg/mL | - | [218] |
| CSDG | 1 w/w | S. aureus, E. coli | Planktonic, Infection model | Colony count, Microscopy | N/R | - | [219] |
| Gelatin film | 0, 0.25, 0.5, 1.0, and 1.5 wt% | E. coli, L. monocytogenes | Planktonic | UFC/mL | 0.25 and 1.5 wt% | - | [220] |
| ZnO-CMC film | 0.5 and 1.0 wt% | E. coli, L. monocytogenes | Planktonic | UFC/mL | 1 wt% | - | [221] |
| Pectin film | 40 mg | E. coli, L. monocytogenes | Planktonic | UFC/mL | N/R | - | [222] |
| Edible film | 0.4% (w/v) | E. coli, B. subtilis | Planktonic | Zone of inhibition | 1% wt. | - | [223] |
CUR has a broad-spectrum antimicrobial activity against viruses, bacteria, and fungi, including resistant and emergent pathogens. However, some species such as Gram-negative bacteria are less susceptible to CUR and aPDT. For those, the combination of CUR with antibiotics has been suggested, especially for antibiotic-resistant strains [220]. CUR showed synergism with polymyxin and protection against the side effects of polymyxin treatment, nephrotoxicity, and neurotoxicity [224]. Nonetheless, the evaluation of synergism requires accurate methods to study drug interaction, considering potential differences between the dose–response relationship of individual drugs and avoiding over- or under-estimation of interactions. For example, while the time–kill curve of C. jejuni treated with both cinnamon oil and ZnO NPs resulted in the over-estimation of synergism between the antimicrobials, the fractional inhibitory concentration index (FICI) method showed no synergism but only an additive effect [225]. The FICI method was not able to detect the synergism between binary combinations of antimicrobials (cinnamon oil, ZnO NPs, and CUR encapsulated in starch) at sub-MIC, which resulted in the non-turbidity of C. jejuni. In turn, mathematical modeling using isobolograms and median-effect curves showed synergism when CUR in starch was combined with other antimicrobials against C. jejuni, with bacterial reductions of 3 log for the binary combination and over 8 log for the tertiary combination. The mathematical modeling suggested that CUR in starch was the main antimicrobial responsible for the synergistic interaction [225].
In addition to the antimicrobial evaluation, in vitro and in vivo studies have demonstrated the cytocompatibility and biocompatibility of CUR in DDSs [108][113][114][115][118][121][144][145][152][156][168][185][192][202][209], suggesting that CUR-loaded DDSs might be safe. Although a plethora of DDSs has been developed to circumvent the hydrophobicity, instability in solution, and low bioavailability of CUR, several studies are still performed with free CUR dissolved in organic solvents [19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][85][86][87][88][89][90]. Furthermore, compared to several in vitro investigations, few in vivo studies using animal infection models and scarce clinical trials have been reported. A randomized clinical trial showed that aPDT mediated by free CUR improved gingivitis in adolescents under fixed orthodontic treatment but did not reduce dental plaque accumulation after 1 month [226]. Clinical improvements after CUR-mediated aPDT were also observed for periodontal diseases, although few studies have evaluated the microbiological parameters [63][89]. Therefore, the improvement of clinical parameters might be due to the anti-inflammatory effect of CUR/aPDT instead of their in vivo antimicrobial activity. Nonetheless, randomized clinical trials evaluating CUR in DDSs against infections are required.
As a note on the future use of CUR, the incorporation of CUR in DDS and other pharmaceutical formulations allows its clinical use especially as an adjuvant agent to conventional antimicrobial agents. Such a combination can be an important weapon in the battle against resistant strains and emergent pathogens. The use of stimuli-responsive (or smart) DDS can also improve CUR delivery and its therapeutic effect on the target tissue. The combination of polymeric and metallic carriers may also enhance the therapeutic activity of CUR. Nonetheless, the degradation of DDS and its clearance from the body are other issues that require further investigation [18]. The evidence produced so far about the antimicrobial activity of CUR in DDSs supports future in vivo and clinical studies, which may pave the way for industrial production.