1000/1000
Hot
Most Recent

Drug delivery systems based on deformable peptides have been widely studied in tumor targeted therapy, for example, proton-driven tumor vaccine composed of deformable peptides for tumor immunotherapy, and an intracellular delivery system of chimeric peptides based on transmembrane peptides for acute liver injury in mice. With the help of software, researchers can take tiny strands of DNA and fold them into complex structures, complete with components such as rotors and hinges that can move and perform tasks, such as drug delivery and cargo handling. A nano-robot based on DNA origami technology can precisely locate tumor tissue and effectively inhibit tumor growth and metastasis.
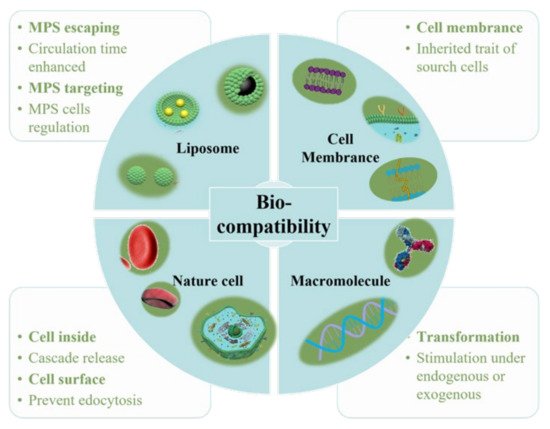
As a “functional molecular device” that can be freely controlled in a liquid environment, nanoparticles have gradually developed into a new generation of multi-functional micro-nano control tools. In particular, it has great application prospects in biomedical fields, such as targeted drug delivery and release, disease diagnosis and so on. Multi-functional nanoparticles work as an important branch of medical micro-nano robots. For the diffusion of nanoparticles, however, micro-nano robots have better initiative, as they control their targeted movement and drug unloading through endogenous or exogenous stimulation [1]. Nanoparticles have excellent performance in in vitro experiments, but they will encounter a complex biological environment at the risk of being eliminated once they enter the physiological environment in the body. Firstly, as a “foreign body”, nanoparticles will be quickly eliminated by the immune system. Secondly, unlike cells, nanoparticles cannot actively sense and target the disease environment, thus limiting the accumulation of nanoparticles in the lesions. Cell membrane–camouflaged nanoparticles comprise a type of synthetic nanoparticle as the core, wrapped with a layer of natural cell membranes, such as red blood cell membranes, immune cell membranes, cancer cell membranes and platelet membranes. Different types of cell membranes will give nanoparticles different biological behaviors [2]. Thus, when constructing the nanoparticle delivery system, biocompatibility must be taken into account. Indeed, the researchers have modified the surface of conventional nanomaterials and optimized their composition in a variety of ways to improve their biocompatibility ( Figure 1 ).
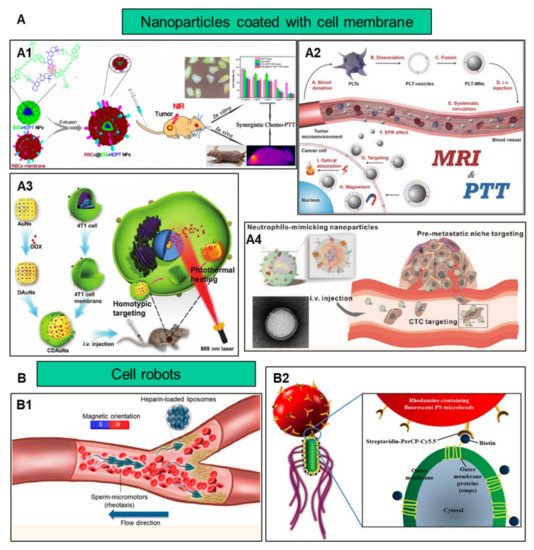
In addition to improving biocompatibility, increasing the target efficiency of nanoparticles will help to further increase nanoparticle efficacy by promoting preferential accumulation at the site of interest. Utilizing the characteristics of different cells, such as chemotaxis of macrophages and the homing effect of cancer cells, nanoparticles and cell characteristics are combined to increase the targeting function of nanoparticles ( Figure 2 ).
Nanoparticles as drug carriers have important research and application value in medicine. The level of drug loading determines the frequency of administration, which is of outstanding significance in reducing clinical costs and side effects and improving the quality of life of patients [9]. According to the existing nano-drug delivery system, the nano-drug carrier system can be divided into four categories as per the drug loading mechanism: molecular-level loading system, loading on the surface of nanoparticles, matrix loading system and cavity loading system [10]. Carriers can be divided into organic carriers and inorganic carriers, which have attracted widespread interest, due to their superior characteristics and promising applications in the field of biomedicine [11]. Nanoscale drug-delivery systems based on organic and inorganic carriers provide an infinite matrix of nanoparticles with different properties. This allows carrier nano-drug delivery systems to perform more complex functions in physiological systems [12]. However, the low drug-carrying capacity of carrier drugs (usually <10 wt%) limits the accumulation of effective drugs. It also promotes the development of carrier-free nano-drug delivery systems (usually greater than 80 wt%).
MPS can present antigens, regulate inflammation, and secrete cytokines to regulate tissue regeneration. Therefore, targeting MPS is an important strategy for directly targeting diseased cells, aimed at increasing the circulation time of liposomes in vivo and avoiding the rapid clearance by MPS. Liposomes’ composition can be optimized by inserting ligands, such as peptides, antibodies, and other biomacromolecules, for disease detection, imaging or treatment.
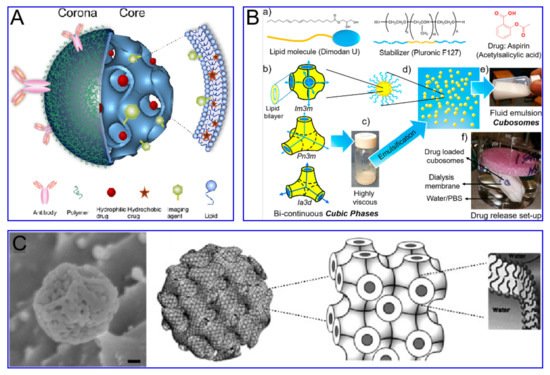
Cubosomes are a lipid bilayer self-assembled by amphiphilic lipid molecules, which further forms a stereoscopic structure with zero mean surface curvature by twisting, cycling and arranging in space according to a cubic lattice. They have been extensively studied in drug delivery ( Figure 3 ). Compared to liposomes, the hydrophobic volume of cubosomes is larger. For the particle size of 100 nm, the volume fraction of the cubosome is 0.59 nm 3 and that of the liposome is 0.18 nm 3. This feature endows the cube with higher drug loading efficiency, especially for poorly water-soluble drugs. Moreover, cubosomes have a rather high viscosity (composite viscosity ranges from 10 4 to 10 5 Pa·s) to a resistance to rupture, ensuring that they are more robust and stable. The cubosome can efficiently load adriamycin and only releases them in an acidic microenvironment, where tumor cells are present, to kill tumor cells. This method not only increases the killing effect of adriamycin on tumor cells, but also reduces the toxic and side effects of adriamycin on normal cells. Cubosomes have been reported to not only promote the proliferation of CD 8+ and CD 4+ T cells, but also induce the secretion of interferon-γ and Ova-specific antibodies. Therefore, cubes can also be used as an effective slow-release delivery system for vaccines. In addition, cubosomes also were used to transport small molecule drugs (both hydrophobic and hydrophilic), peptides, proteins, nucleic acids and imaging agents into cells through oral, intravenous, transdermal and mucosal administration.
Nanomaterials coated with cell membranes are regarded as a promising method of biomimetic particle engineering [16]. Natural cell derivatives, such as extracellular vesicles and membranes, can inherit many of the properties of their source cells. Therefore, by coating nanoparticles with these derivatives, the nanoparticles not only have natural biocompatibility, but also have functions similar to those of their source cells [17]. So, this top-down engineering approach can be applied to the development of new therapeutic strategies [18]. Membranes that can be used to coat nanomaterials have been reported to involve the red blood cells [19], immune cells [20], platelets [21] , stem cells [22], macrophages [23] and cancer cells [24]. These membrane-interfacing nanomaterials have been reported for use in targeted therapy, vaccination, virus detection and many other fields.
Using the body’s own cells as a vehicle is advantageous because autologous cells are not only incomparably biocompatible, but they also have a built-in mechanism to move between tissues and navigate long distances around the body [25]. In order to avoid damage to the carrier cells by the loaded drugs, the drug loading rate of the cells must be limited [9]. To solve this problem, researchers wrap drugs in nanoparticles and deliver them to the lesion site either by attaching them to the cell surface or by endocytosis into the cell. Then, the loaded drugs can be released in response to either endogenous or exogenous stimuli [26]. Cells that have been reported for use as nanomaterial carriers include stem cells [27], leukocytes [28], red blood cells [29], and T cells [30]. Methods of endogenous stimulation include pH, reactive oxygen species (ROS), enzyme, hypoxia and so on [31]. The exogenous stimuli include near infrared radiation (NIR), magnetic field, ultrasound, etc. [32]. When used as drug carriers, erythrocytes have high encapsulation rates and long-term sustained release [33]. White blood cells (including monocytes, neutrophils, and lymphocytes) can actively cross biological barriers, such as tissue endothelium and the blood–brain barrier; they also have a tendency of chemoattraction toward the diseased site to inhibit infection, inflammation and tumor growth [34]. Because of this unique feature, they have the potential to carry drugs to other parts of the body that are inaccessible or difficult to reach through traditional drug delivery strategies.
The occurrence and metastasis of malignant tumors are usually caused by the immune escape of tumor cells. Cancer cells develop complex mechanisms to counteract or evade immune surveillance; for example, the overexpression of CD47 on the surface of breast cancer cell membranes may prevent them from being eliminated by the immune system. Nanoparticles encapsulated by cancer cell membranes are endowed with homotypic targeting properties. The study demonstrated that, compared with the uncoated tumor cell line MDA-MB-435 membrane, there was a significant accumulation of cancer cell membrane-coated nanoparticles in cancer cells [35]. Liu et al. constructed mesoporous silica nanoparticles with CaCO 3 and cancer cell membranes, and the results showed that the cancer cell membrane coating contributed to the stability of the particles and the ability to accumulate at the tumor site [36]. Another group used lung cancer cell H460 membranes to bind two peptides, PD-L1 inhibitory peptide (TPP1) and MMP2 substrate peptide (PLGLLG), to coat superparamagnetic iron oxide nanoparticles through the homotypic effect of tumor cell membranes and the specific digestion of tumor-specific enzyme MMP2. The TPP1 peptide was delivered and released into the tumor micro-environment, showing a promising tumor treatment platform [37]. Compared with bare particles, nanoparticles wrapped in MCF-7 cell membranes significantly boost the absorption of nanoparticles by homologous cells [38]. In addition to targeting primary tumors, the use of homotypic targeting strategies can also deliver nanoparticles to metastatic tumors. The surface of 4T1 breast cancer cell membranes contains proteins and adhesion molecules related to metastasis and homotypic binding. These characteristics enable the cancer cell membrane-coated nanoparticles to effectively target metastatic breast cancer [5].
Compared with nanoparticles camouflaged by RBC membranes, those decorated with white blood cell membranes can not only prolong circulation in the body, but also actively target functional molecules on inflammation sites and cancer cell membranes. However, there are some issues that need to be considered. For example, as white blood cell membranes are mostly derived from immortalized cells, their biocompatibility is not as good as their red blood cell counterparts. In addition, the expression of specific histocompatibility complex molecules (MHC) on the leukocyte membrane may give rise to immunogenicity [39].
Integrating two or more kinds of cell membranes on the surface of nanoparticles can create unique biological properties of nanoparticles. For example, the RBC membrane and the cancer cell membrane are integrated to improve the efficiency of drug delivery. The platelet membrane and the cancer cell membrane are combined and modified with antibodies to increase the binding ability of cancer cells, which can reduce the interaction of homologous white blood cells and facilitate the separation of specific cancer cells. The gold nanowires are coated with the platelet membrane and the RBC, which can perform two different tasks at the same time: the platelet targets bacteria, while the RBC targets and neutralizes the toxins produced by the bacteria [40]. Another case showed that the cancer cell-RBC hybrid membrane-coated doxorubicin-loaded gold nanocage exhibits highly efficient accumulation in tumor sites due to the homologous targeting of the cancer cell membrane and decreased clearance due to the RBC membrane [41].
Although some progress has been made in nanoparticles camouflaged by cell membranes, some issues still exist. First of all, the source of cell membranes is very limited, and the separation and extraction steps are cumbersome, with a low yield. Secondly, the structure on the cell membrane is very complicated, and some components may induce an immune response. Finally, the control of cell membrane quality and safety also poses a problem. Nanoparticles for good cell membrane decoration require multidisciplinary cooperation.
Mesoporous carbon nanoparticles are widely used to construct nano therapeutic systems, due to their excellent physical and chemical properties. Compared with traditional silica-based nanocarriers, the hydrophobicity of hollow mesoporous carbon nanospheres (HMCNs)–based nanocarriers can achieve higher drug loading efficiency. The high loading capacity should be attributed to the hollow structure of HMCNs, its π–π stacking with the drug and the non-covalent interaction of electrostatic attraction [42]. By introducing near-infrared light irradiation or H 2O 2, the movement of HMCNs can be promoted, and the number of HMCNs attached to the surface of cancer cells will grow, which is conducive to improving the efficiency of drug delivery [43]. In the DOX/HMC-Au@PEG system constructed by Zhao et al., the loading capacity of DOX is as high as 40.6%, and the system displays dual-triggered drug release characteristics of redox and NIR [44]. Gui et al. developed a simple and efficient strategy for preparing fluorescent hollow mesoporous carbon spheres (HMCS). Ten drugs commonly used in cancer treatment—including DOX, 5-FU, PTX, CTX, MB, SCC, Ce6, DDP, CUR and QUE—were successfully incorporated into HMCS, with a maximum load efficiency of 42.79 ± 2.7% [45]. Importantly, when combined with 980 nm laser irradiation, it was found that microwaves could improve the photothermal effect produced by HMCS and check the growth of tumor cells. Carbon nanotubes (CNTs) are a carbon allotrope, which have the characteristics of resonance light luminescence, strong NIR optical absorption and Raman scattering, and can be used for cancer multi-modal imaging and treatment [46]. In addition, CNTs can also enhance the anti-tumor effects of chemotherapeutics by improving the accuracy and efficiency of drug delivery [47]. However, the potential long-term toxicity also limits the practical application of CNTs.
An inherent problem of carrier nanomedicine is that its drug-carrying capacity is still low (usually <10 wt%), which hinders the accumulation of effective drugs and the therapeutic effects of drugs. The drug inertness of the nanocarrier and the excessive chemical treatment during the preparation process will bring potential harm to the body [48][49]. In order to avoid these hazards, carrier-free nanomedicines, whose nanomaterial matrix is mainly composed of active pharmaceutical ingredients, were developed. The drug loading of all these types of nanomedicines is usually higher than 80 wt%. As per different building blocks, we divide SDND into pure nano-drugs and drug-drug conjugates for discussion [50][51].
Drug–drug conjugates are usually self-assembled from multiple drugs. These drugs can be complete therapeutic ingredients or contain small amounts of non-therapeutic ingredients (synthetic polymers or natural biopolymers). In the previous article, we discussed the organic carrier nano-drug delivery system, so next, we will mainly delve into the drug–drug-combined pure nano-drug containing complete therapeutic ingredients. Yan’s research group synthesized an amphiphilic drug conjugate (ADDC), using the hydrophilic anticancer drug irinotecan (Ir) and the hydrophobic anticancer drug chlorambucil (Cb) through hydrolyzable ester bonds [52]. After cell internalization, the ester bond between the drugs was hydrolyzed, releasing free Ir and Cb. Compared with the free drug, this drug showed a longer blood retention half-life, and at the same time effectively overcame the multidrug resistance (MDR) of tumor cells, exhibiting excellent anti-cancer activity. In another article, the hydrophilic floxuridine (FUDR) and the hydrophobic anti-angiogenic drug pseudolaric acid B (PAB) were conjugated to obtain the ADDC compound FUDR-PAB via one-step esterification and conjugation, which also manifested excellent anti-cancer activity [53]. However, the coupling of hydrophilic and hydrophobic drugs limited the application of ADDC. Furthermore, the changes in the molecular structure of the drug during the synthesis process may have a potential impact on the anti-cancer efficacy and pharmacokinetics [50].