Studies have demonstrated that men with Prostate Cancer (PCa) harboring BRCA2/BRCA1 genetic aberrations, are more likely to have worse disease and a poorer prognosis. A mutation in BRCA2 is known to confer the highest risk of PCa for men (8.6 fold in men ≤65 years) making BRCA genes a conceivable genomic biomarker for risk in PCa. These patients can be treated successfully with a group of drugs called ‘PARP inhibitors’. This paper examines the prognostic, clinical and therapeutic role of BRCA2/BRCA1 mutations across the evolution of PCa.
1. Introduction
Prostate Cancer (PCa) was the second most common cancer diagnosis made in men (14.1%) and the fifth leading cause of death (6.8%) worldwide in 2020
[1]. Population expansion and improved life expectancies across the globe are set to contribute to an increase in PCa
[2] rendering it an major worldwide health concern. While there has been an emergence of novel treatments in the last ten years, even now PCa is a major source of cancer deaths in males
[3]. Older age is the chief risk factor with greater than three quarters of all PCa detection made in men over the age of 65 years
[4]. Family history and genetic predisposition such as pathogenic variants
BRCA1/BRCA2 have also been identified as important risk factors
[5][6]. Other genes important for DNA repair can also play a role if mutated (see
Table 1). These extend to the DNA mismatch repair genes
MLH1,
MSH2, PMS2, MSH6, and
EPCAM [7] that play a role in Lynch syndrome. When functioning correctly
BRCA1/BRCA2 are important tumor suppressor genes with multiple functions including transcription and complex cell cycle control
[8]. However, their primary role is in the repair of DNA double stranded breaks (DSB) through the initiation of homologous recombination (HR). Failure of this system due to mutations in these genes can predispose an individual to malignancies such as breast, ovarian, pancreatic and prostate cancers
[9]. A mutation in
BRCA2 is known to confer the highest risk of PCa for men (8.6 fold in men ≤65 years), with
BRCA1 demonstrating increased risk, albeit to a lesser extent (3.5 fold)
[10]. These genes have attracted a lot of research attention however their role in the clinical assessment and treatment of PCa remains complex. This review seeks to address the role of
BRCA2 and
BRCA1 mutations in PCa in terms of the clinical and therapeutic implications starting with their discovery in the 1990s.
Table 1. List of identified germline genes that have been implications in advanced prostate cancer from seminal work from Pritchard et al.
[11].
| Gene |
% Prevalence Pathological Gene Mutation in Metastatic PCa |
| BRCA2 |
5.53% |
| CHEK2 |
1.87% |
| ATM |
1.59% |
| BRCA1 |
0.87% |
| GEN1 |
0.46% |
| PALB2 |
0.43% |
| RAD51D |
0.43% |
| ATR |
0.29% |
| PMS2 |
0.29% |
| BRIP1 |
0.18% |
| FAM175A |
0.18% |
| MSH2 |
0.14% |
| MSH6 |
0.14% |
| RAD51C |
0.14% |
| MRE11A |
0.14% |
2. Evolution of Knowledge of BRCA Genes Risk in Prostate Cancer
Dr. Mary-Claire King was the first to demonstrate that a single gene on chromosome 17q21 (then named
BRCA1) was responsible for breast and ovarian cancer in many families in the 1990s, thus demonstrating a heredity component in these cancers and proving that gene mutations could predict vulnerability to these diseases
[12]. Her discovery revolutionized the study of cancer genetics and has proved seminal in investigating heritability of many diseases and cancer subtypes
[13]. The impact of germline mutations of
BRCA1 and
BRCA2 in breast and ovarian cancer are now well defined.
BRCA1 and
BRCA2 mutations result in 70% risk of developing breast cancer by the age of 80
[14], compared to 12% of the unaffected population. These genes impact early diagnosis, prevention and therapeutics in breast cancer, and have led to the refinement of national and international genetic screening guidelines. The clinical impact of the role of DNA damage repair genes is still evolving in PCa, although it likely mirrors the path of hereditary breast and ovarian cancer
[13]. Indeed much of the initial observations on PCa heritability arose via a circuitous route from breast cancer studies examining
BRCA1 and
BRCA2. The recognized association of breast cancer with PCa in families has been reported since the 1970s
[15]. Important Icelandic studies in the 1990s reported that male relatives in breast cancer families were noted to have a 2–3 fold risk of PCa
[16]. Further studies showed that males who harbored a germline
BRCA1 and
BRCA2 mutation, identified via a family history of breast cancer, were reported to have an increased the risk of PCa by three-fold and seven-fold, respectively
[17][18]. Therefore, from breast cancer studies, it was first derived that men with
BRCA1 and
BRCA2 mutations were at higher risk for PCa.
3. BRCA Mutations in Prostate Cancer
In analyzing PCa genetics, it is critical to distinguish between localized versus high risk and metastatic disease. Firstly, owing to widespread adoption of PSA, the majority of new diagnosis of PCa are localized low-grade disease with excellent prognosis. These are clinically distinct from comparatively fewer diagnoses of advanced metastatic PCa
[19], who are recognized to have the potential for poor disease outcomes. Several studies have shown a different genomic/genetic landscape in metastatic Castrate Resistant PCa (mCRPC) compared to localized PCa
[20][21]. It is problematic to derive meaningful clinical predictions when examining PCa as a whole given the broad clinicopathological heterogeneity of the disease. This can be illustrated by germline mutations in
BRCA2 having been previously underappreciated as a driver of hereditary PCa. Genomic profiling of PCa was first extrapolated from material acquired at unselected prostatectomies and therefore genetic defects were considered to be rare
[22]. Therefore, ascertainment bias has thwarted the reporting of the true prevalence of pathogenic genetic mutations in advanced metastatic PCa.
3.1. Prevalence of BRCA Mutations in Prostate Cancer
Advanced disease is associated with higher proportion of germline mutations in DNA-repair genes in patients with PCa, with 4.6% prevalence identified with germline mutations in localized cancer and 11.8% to 16.2% witnessed in the metastatic setting
[11]. Pritchard et al. reported observing incidence rates of germline
BRCA2 (5.35%) and
BRCA1 (0.87%) in the advanced PCa setting
[11]. Robinson et al. performed genomic profiling on mCRPC biopsy samples from 150 affected individuals with metastatic disease with complete integrative clinical sequencing results (whole exome, matched germline, and transcriptome data). They identified
BRCA2 of both the somatic and pathogenic germline alterations in 19/150 (12.7%) cases. Eight affected individuals (5.3%) harbored pathogenic germline
BRCA2 mutations with a subsequent somatic event that resulted in biallelic loss
[20]. These results report are similar incidence of germline
BRCA2 to the Prichard et al. study. Another study observed germline and somatic DNA mutations in 944 men with both early and advanced PCa
[23]. The most frequently mutated gene was
BRCA2, pathogenic variants of which were identified in 11.4% of distinct samples with pathogenic
BRCA1 variants identified at a frequency of 1%. Unfortunately, a limitation of the study was their inability to distinguish between germline and somatic mutations and therefore these figured lack granularity on frequency of germline and somatic mutations. This compares with 1–3% of unselected early PCa’s which harbor
BRCA2 germline mutations
[24].
3.2. BRCA Mutations in Prostate Cancer by Mutation Type
Dall et al., referenced above, provided further granularity on the nature of these mutations, particularly in
BRCA2 mutation carriers
[23]. They examined both germline and somatic mutations from 944 unique men, sequencing samples using Foundation medicine. From the 105 samples identified as having alterations in
BRCA2, the majority were truncating mutations (70%). Twenty-eight samples (26%) had complete deletion and 3 samples had point mutations. Of the 5 prostate samples with biallelic alterations in
BRCA2, each allele demonstrated different frequencies. The majority of the 10 samples with identified
BRCA1 alterations (80%) were truncating and all were monoallelic with allele frequencies of 0.4 to 0.8, suggesting a germline mutation. Of note, 60% had coincident TP53 alterations, an occurrence also described in
BRCA1-associated breast cancers
[23]. The varying genetic profile between localized PCa and metastatic lesions may be the result of tumor development beneath the discriminating pressure of prior treatments, such as Androgen Deprivation Therapy (ADT), Androgen Receptor Signaling Inhibitor (ARSi) or chemotherapy. However, it is of course possible that certain mutations might be already present in primary tumors and might expand during the development of metastatic disease and thus become the driver the of disease
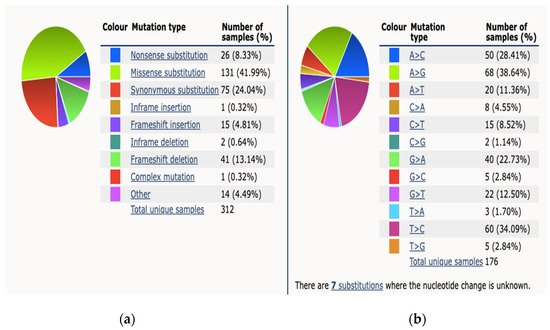
[6]. The Catalog Of Somatic Mutations in Cancer (COSMIC) reports 312 somatic BRCA2 mutations from 4093 samples tested across all subtypes of PCa, a percentage prevalence of 7%
[25]. The breakdown mutation type and observed substitution mutations is demonstrated in
Figure 1.
Figure 1. This figure demonstrates mutation distribution for BRCA2 gene as extracted from COSMIC database 2021. (a) An overview of the types of mutations observed (b) a breakdown of the observed substitution mutations.
4. Current Treatment Options for BRCA Mutated Prostate Cancer
For the majority men diagnosed with PCa, their outcomes are encouraging; with 98% of men in the USA and 83% in Europe alive 5 years after diagnosis
[3]. Early stage PCa can be definitively treated with radiation treatment or surgery, and given the indolent nature of some PCa’s there can be a place for surveillance
[26]. PCa will recur in approximately 20% to 30% of unselected men (i.e., unknown mutation status) treated for localized PCa. Since Huggins and Hodges won a Nobel Prize in 1966 for their work describing the relationship between testosterone and PCa, Androgen-Deprivation Therapy (ADT) has continued to be an important component in the treatment of advanced PCa
[27]. Despite the majority of men with metastatic PCa at first responding to ADT, the average duration of response in metastatic castration-sensitive PCa (mCSPC) spans from 24 to 36 months. Inevitably their PCa stops responding to this therapy, a condition known as castration-resistant PCa (CRPC)
[26]. The average survival for patients with mCRPC is less than 2 years
[28] despite new therapeutic strategies for CRPC being offered to patients, such as new combinations and sequences of second-generation antiandrogen therapy (enzalutamide, abiraterone, apalutamide) or second line chemotherapy (cabazitaxel), which have shown notable benefit for patient survival
[26].
There are three large phase clinical trials showing efficacy of Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) in the advanced setting detailed below, however there is otherwise a lack of high level evidence surrounding the distinct treatment of
BRCA mutation carriers within the current treatment framework. There is particularly a lack of evidence guiding radical treatment of localized PCa for
BRCA mutation carriers
[29][30]. Building on the progress achieved in the advanced PCa setting, combined with the knowledge that
BRCA mutant PCa is more aggressive, it is iterative to explore the role of more rigors treatment regimes in the localized high risk setting. This mirrors similar studies which have been performed in the breast cancer space. Recent phase 3 double blinded clinical trial by Tutt et al. demonstrated that among patients with high-risk, early breast cancer with an identified germline
BRCA1 or
BRCA2 mutations, treatment with a PARPi (Olaparib) after completion of local treatment and chemotherapy was associated with significantly longer survival free of invasive or distant disease versus placebo
[31]. Clinical trials are underway examining if there is a role for neo-adjuvant or adjuvant treatments with PARPi for patients with a
BRCA mutation
[32][33].
4.1. PARPi for BRCA Mutated Prostate Cancer
In vitro studies have demonstrated that PCa cells harboring
BRCA1 and
BRCA2 mutants are up to 1000 times more sensitive to PARPi
[34][35]. At present there are two PARPi, Olaparib and Rucaparib which have demonstrated efficacy in patients with genetic mutations in PCa, namely
BRCA2,
BRCA1, or
ATM mutations
[12][36]. Olaparib has known survival benefit for patients diagnosed with breast cancer or High Grade Serous Ovarian Cancer (HGSOCa) with homologous recombination deficiency
[31][37][38][39]. PROfound was a phase III randomized controlled trial comparing a PARPi (Olaparib) with a Androgen Receptor Signaling Inhibitors (ARSi) in men with mCRPC
[36]. Men who were eligible were men with mCRPC who’s diseased had not responded or stopped responding to treatment with an ARSi, namely Abiraterone or Enzalutamide. Men with three identified gene alterations (
ATM, BRCA1 and
BRCA2) were treated with the PARPi or an ARSI (either Abiraterone or Enzalutamide). Another subgroup was formed of men with twelve additional predetermined gene defects, who received a PARPi or an ARSi as above. When the overall population was assessed, the patients treated with PARPi had improved outcomes compared to the ASRi group (PFS 5.8 months vs. 3.5 m; HR, 0.49; 95% CI, 0.38 to 0.63;
p < 0.001). The advantage was most marked in the first group (PFS 7.4m vs. 3.6m; HR for progression or death, 0.34; 95% (CI), 0.25 to 0.47;
p < 0.001). Similarly, TRITON2 a phase II open label study, investigated the benefit of Rucaparib in PCa for men with mCRPC with deleterious
BRCA mutation (germline and/or somatic) previously treated with ARSi and a taxane based chemotherapy. The populations included 115 patients with a
BRCA1 alteration (n = 13) and
BRCA2 alteration (n = 102). Confirmed ORRs were 43.5% (95% CI, 31.0% to 56.7%; 27 of 62 patients) and 50.8% (95% CI, 38.1% to 63.4%; 33 of 65 patients), respectively. The confirmed PSA response rate was 54.8% (95% CI, 45.2% to 64.1%; 63 of 115 patients). ORRs were similar for patients with a
BRCA1 or
BRCA2 alteration, while a higher PSA response rate was observed in patients with a
BRCA2 alteration. The outcomes of this study resulted in its approval by the FDA
[12]. There are currently over twenty clinical trials involving PARPi in combination with other treatment modalities listed on
clinicaltrials.gov (accessed on 13 November 2021) in the advanced PCa space in an attempt to establish optimum treatment sequence. This has been summarized and tableted by Sigorski et al.
[34]. Of note, eligibility for inclusion in clinical trials for Olaparib and Rucaparib required the identification of a pathogenic mutation by either germline and/or somatic testing
[12][40].
4.2. Chemotherapy for BRCA Mutated Prostate Cancer
BRCA2 mutations have been associated with a higher likelihood of response to carboplatin-based chemotherapy than non-
BRCA2-associated PCa in CRPC
[41], which is somewhat foreseeable given Carboplatin is a standard treatment for
BRCA1 and
BRCA2 patients in ovarian and breast cancer settings. Platinum-based chemotherapy, alkylating DNA, induces genomic strand breaks that may be translated in a synthetic lethality in tumor cells with DNA Damage Repair (DDR) mutations.
At present, only two taxane-based chemotherapies, namely Docetaxel and Cabazitaxel, have shown efficacy in the advanced PCa setting
[26]. Gallagher et al. reported half of
BRCA carriers had a PSA response to taxane-based chemotherapy, suggesting that it is an active therapy in these individuals, with 71% (54/76) of patients responding to treatment, with no significant difference between carriers (57%) and non-carriers (72%) (absolute difference 15%; 95% CI, 23% to 53%;
p = 0.4)
[42]. Trials assessing the efficacy of platinum-based chemotherapies in mCRPC patients with
BRCA mutations are ongoing
[43][44]. Platinum chemotherapy generates interstrand cross-links that can only be adequately repaired by HR based DNA repair, and consequently BRCA1 and
BRCA2 mutated cells are highly sensitive to platinum chemotherapy both in vitro and in vivo
[45]. It is also a matter of considerable interest whether men with identified
BRCA mutation in PCa would benefit from chemotherapy early in their disease course.
4.3. Androgen Receptor Signalling Inhibitors for BRCA Mutated Prostate Cancer
There are a number of second-generation antiandrogen therapies (enzalutamide, abiraterone, apalutamide and darlutamide) with proven efficacy in PCa
[46][47][48][49]. Antonarakis et al. evaluated the clinical significance of DDR mutations in 172 mCRPC patients receiving first-line ARSi and found that
ATM-BRCA1/2 carriers had a trend towards longer progression-free survival (PFS) than noncarriers (15 vs. 10.8 months,
p = 0.090)
[50]. However Annala et al. reported that defects in
BRCA2 and
ATM were strongly associated with poor clinical outcomes independently of clinical prognostic factors and circulating tumor DNA abundance when treated with abiraterone or enzalutamide
[51].
BRCA2 and
ATM carriers had a significant shorter PFS than noncarriers (3.3 vs. 6.2 months,
p = 0.01) when treated with first-line ARSi. Prospective validation in larger patient cohorts will be required given the conflicting results in the literature.
5. BRCA as a Biomarker for Prostate Cancer Risk in Special Populations
BRCA variation across ethnicities has been well established across multiple studies in Ashkenazi Jewish, Polish, Icelandic populations and Asian population
[52][53][54][55]. The issue of ethnic-specific BRCA variation has important clinical implications
[56] in guiding prevention and treatment of BRCA-related cancer. Selected below are ethnic groups where specific founder mutations in BRCA have been established as a significant biomarker for risk for the population group effected.
5.1. Icelandic Population
As noted above, Icelandic studies in the 1990s reported that male relatives in breast cancer families were noted to have a 2–3 fold risk of PCa
[16]. Initial studies from Iceland reported that PCa occurring in BRCA2 mutation carriers were more aggressive than those in non-carriers, and had poorer survival
[16]. It was discovered that a ‘999del5′ mutation in the
BRCA2 gene explains a substantial proportion of familial risk of breast cancer in Iceland. This single
BRCA2 mutation accounts for 7–8% of female breast cancers and 40% of male breast cancers there
[57]. The BRCA2 999del5-associated cancer risk is most probably due to haploinsufficiency
[58]. Clinical practice guidelines for
BRCA1 and
BRCA2 direct that genetic testing needs to be considered more carefully for women from populations with a small spectrum of founder mutation, with a founder effect, such as in Iceland
[59]. Similar founder mutations have been identified in the polish population
[60] (see
Table 2).
5.2. Ashkenazi Jewish Populations
In Ashkenazi Jews,
BRCA mutations are found in up to 5.2% of unselected patients with PCa
[61]. Another study demonstrated that more than 2% of Ashkenazi Jews carry these germline mutations, specifically the mutations in
BRCA1 involving the deletion of an adenine and guanine (185delAG) and the insertion of a cytosine (5382insC), and a mutation in
BRCA2 involving the deletion of a thymine (6174delT). These carriers have a 16% chance (95% CI, 4–30%) of developing PCa by the age of 70 and 39% by the age of 80
[62]. NCCN guidelines reflect this risk by recommending germline testing for any persons of Ashkenazi Jewish descent with a diagnosis of PCa, regardless of local or metastatic status
[19]. The Philadelphia Prostate Cancer Consensus Conference guidelines 2019 however made separate recommendations for Ashkenazi Jews with metastatic PCA (castration resistant or castration sensitive disease (recommend genetic testing) versus men with nonmetastatic PCA (consider testing)
[7].
5.3. Black African Men
An analysis of population-based cancer registries found that the incidence of PCa was higher in black men than in white men
[63]. It has also been reported that African-American men have a higher lifetime risk of developing (18.2% vs. 13.3%) and dying from (4.4% vs. 2.4%) PCa compared to Caucasian-American men
[64]. While there is a scarcity of evidence, a small study showed that African American men are less likely to habor BRCA mutations when compared to the Pitchard et al. data
[11][65]. Further analysis are underway to explain these clinical disparities. Hypothesis include difference in tumor location within the prostate that may reflect different PCa subtypes, differences in gene expression and/or treatment disparities and access to health care
[66][67][68].
Table 2. BRCA1/2 founder mutations.
| BRCA2/BRCA1 |
Mutation |
Population |
References |
| BRCA1 |
185delAG (c.68_69delAG) |
Ashkenazi Jewish |
Tenner et al. [69] |
| BRCA1 |
5382insC (c.5266dupC) |
Ashkenazi Jewish |
|
| BRCA2 |
6174delT (c.5946delT) |
Ashkenazi Jewish |
|
| BRCA2 |
999del5 (999del5-2T) |
Icelandic Population |
Tulinius et al. [57] |
| BRCA1 |
5382inC (c.5266dupC) |
Polish Population |
Kowalik et al. [60] |
| BRCA1 |
300T > G (c.181T > G) |
Polish Population |
|
| BRCA1 |
185delAG (c.68_69delAG) |
Polish Population |
|
| BRCA1 |
4153delA (c.4035delA |
Polish Population |
|