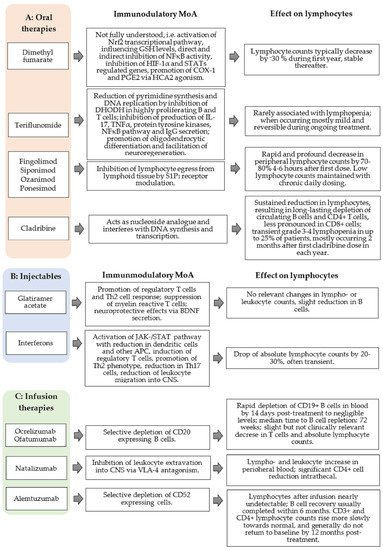
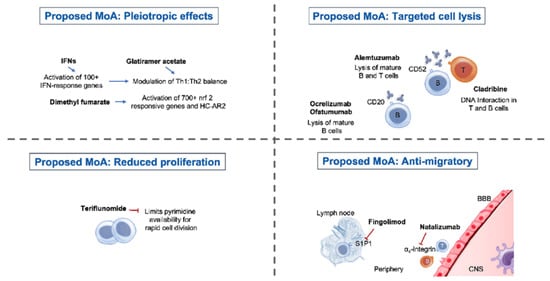
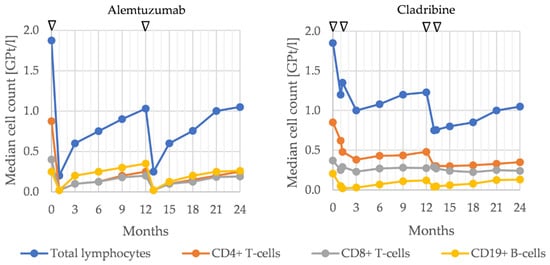
Mechanism of Action and Impact on Lymphocyte Counts
Fingolimod, siponimod, ozanimod, and ponesimod are structural analogs of natural sphingosine phosphate
[77]. In a phosphorylated state, fingolimod binds to four of the five known S1P receptors (S1PR
1 and S1PR
3–5)
[78][79]. Siponimod, ozanimod, and ponesimod exhibit selective affinity for type 1 and 5 of the S1P receptors, leading to a lower risk of adverse events, such as bradycardia and vasoconstriction, mainly induced by S1PR
3 activation.
Binding with high affinity to S1PR
1 expressed on lymphocytes, lymphocyte egress from lymphoid tissues into the peripheral compartment is inhibited by all approved S1P receptor modulators, preventing the infiltration of auto-aggressive lymphocytes into the CNS (
Figure 1A)
[80][81][82]. Initial receptor activation is, paradoxically, followed by S1PR
1 functional antagonism. Accordingly, receptors are internalized and degraded, thus rendering lymphocytes unresponsive to the normal S1P gradient, which represents the obligatory signal that would ordinarily allow them to egress from lymphoid tissues
[81][83][84]. Additionally, binding to S1P receptors expressed in the CNS (S1PR
1/5) promotes a modulating effect on neurogenesis, neural function, and migration
[85][86]. Fingolimod binds to S1PR
1/3 on smooth muscle and endothelial cells, which influences vascular homeostasis and vascular permeability. Furthermore, fingolimod induces a negative chronotropic effect via S1PR on atrial myocytes
[87][88].
As S1PR modulators inhibit CCR7+ lymphocyte egress from secondary lymphoid organs, resulting in a profound decrease in naive and central memory T cells and memory B cells in the periphery
[89][90]. Treatment with fingolimod significantly decreases the absolute numbers of all major lymphocyte subsets, except for NK cells. The reduction is most pronounced within T helper and B cell populations
[91]. Dramatic reductions within the naïve and central memory T cell populations can be found
[91]; the reduction is less pronounced among effector memory cells. The number of regulatory T cells (Tregs) also decreases, but to a lesser extent than other T cell populations, resulting in a relative preservation of Tregs with a memory phenotype
[91]. In summary, within T cells, naïve and central memory cells are most profoundly affected by a fingolimod-induced reduction, whereas memory Tregs are relatively preserved.
A dose-dependent decrease in total peripheral lymphocytes by 70–80% can be observed, and most fingolimod-treated patients reach grade 2–4 lymphopenia after starting therapy. Grade 4 lymphopenia is a common adverse event occurring in 15–20% of patients
[92][93]. In a German and Swedish cohort of fingolimod-treated patients with a low baseline lymphocyte count, women with a low body mass index were suggested to have a higher risk of lymphopenia
[92]. A history of treatment with any IFN-β was significantly more frequent in patients who experienced lymphopenia
[94]. This is because the IFN-β family influences the production of cytokines by lymphocytes and are considered to be related to myelosuppressive activities
[95]. A study by Ohtani et al. showed that a low lymphocyte count at baseline and a treatment history of any IFN-β therapy is associated with grade 4 lymphopenia during fingolimod treatment
[94]. Lymphocytes and their subsets return to the normal range around 1–2 months after treatment discontinuation
[96]. Different studies discuss efficacy depending on T and B cell decreases during fingolimod therapy. Current real-world data show a wide range of peripheral lymphocyte counts during treatment, depending on the individual distribution of CD4+ and CD8+ T cells, CD19+ B cells, and NK cells. While peripheral CD4+ T cells and CD19+ B cells are markedly reduced by S1PR
1 therapy, CD8+ T cells and NK cells are less affected and less relevant to variations in lymphocyte counts in individual patients
[97].
It is assumed that withdrawal of fingolimod results in overexpression of lymphocytic S1PR
1 leading to lymphocyte egress from lymph nodes and promoting disease rebound after treatment discontinuation
[98]. Autopsy results from a patient who died after severe rebound relapse revealed increased S1PR
1 immunoreactivity on hypertrophic astrocytes in tumefactive plaques, indicating that the withdrawal of fingolimod results in astrocytic overexpression of S1PR
1 [99][100]. Due to the increased risk of more intense lymphopenia during fingolimod therapy, different treatment regimen alternatives have been assessed. However, the change from conventional therapy to intermittent dosing carries a risk of rebound, and the efficacy of an alternate-day fingolimod administration was not effective enough to inhibit disease activity
[101][102].
Siponimod leads to a dose-dependent reduction of peripheral lymphocytes to 20–30% of baseline (median nadir approximately 0.56 GPt/L), with a recovery to the normal range within 10 days in 90% of patients after treatment discontinuation
[103]. However, in some patients, lymphocyte recovery can take up to 3–4 weeks. In the pivotal phase III EXPAND study, grade 4 lymphopenia was observed in 1% of patients
[103].
There are insufficient real-world data of lymphocyte count during ozanimod treatment. Combining data from the RADIANCE and SUNBEAM trials enabled a comparison of ozanimod to fingolimod, and analysis showed a higher absolute mean lymphocyte count (difference in means 0.4 GPt/L) during ozanimod treatment compared with fingolimod treatment
[104]. During the RADIANCE study, ozanimod treatment led to dose-dependent suppression of absolute lymphocyte counts to <0.2 GPt/L in four participants (3.3%). These reductions were transient and not associated with infections or treatment discontinuation
[105]. Early clinical studies of ponesimod therapy show an overall reduction of absolute lymphocyte count, compared to baseline, of about −1.3 GPt/L. Ponesimod treatment led to a marked reduction in overall T and B cell counts. Specifically, the number of CD4+ cells showed a significant drop, whereas CD8+ and NK cells were less affected
[106]. Similar to siponimod and ozanimod, reliable real-world data for ponesimod are not yet available due to recent regulatory approval.
Taken together, data on studies of siponimod, ozanimod, and ponesimod show a lower risk of higher-grade lymphopenia than for fingolimod, and this might be considered when selecting treatment alternatives where the desire is for fewer side effects.
Recommended Monitoring
Before starting treatment with S1PR-modulators, chronic active infections should be excluded. Specifically, VZV status should be defined, and the evaluation of hepatitis B, C, and HIV should be considered. In the absence of VZV antibodies, patients should be immunized with VZV vaccine prior to treatment, which can be started four weeks after vaccination at the earliest.
Four weeks after the commencement of S1PR-modulators, a complete blood count should be performed (Table 2). Subsequent laboratory intervals can be increased to 3–6 months in the case of normal lymphocyte and leukocyte counts. In the case of peripheral lymphopenia < 0.2 GPt/L (confirmed by a second test after two weeks), S1PR therapy should be discontinued until lymphocyte counts reach levels > 0.6 GPt/L (Table 1).
In the case of acute infection, diagnostic and therapeutic measures should be adopted immediately, especially concerning viral herpetic infections (e.g., VZV infection or reactivation, Herpes simplex virus (HSV)-encephalitis), mycotic (e.g., cryptococcal meningitis), or bacterial infections (e.g., atypical mycobacteria). A higher risk of infections can be assumed considering the underlying mechanism of action. However, trial results suggest that for S1PR modulators, there is no direct correlation between absolute peripheral lymphocyte count and the likelihood of infective complications
[93].
The risk of PML during S1P receptor modulator therapy is lower than that for natalizumab
[107]. In most of the known cases, a ‘carry over’ mechanism following prior natalizumab therapy is assumed. There was no correlation to peripheral lymphopenia
[107]. Frequent MRIs should be performed to assess the potential risk of PML, in addition to standard MRI MS monitoring. Regular evaluation of JCV-serostatus should be considered.
Efficacy of vaccination can be limited during, and up to two months after, therapy discontinuation. Immunization with live, attenuated vaccines should be avoided during S1PR modulator therapy.