1000/1000
Hot
Most Recent

Siloxanes are adaptable species that have found extensive applications as versatile materials for functionalising various surfaces and as building blocks for polymers and hybrid organic-inorganic systems. The primary goal of this review is to report on and briefly explain the most relevant recent developments related to siloxanes and their applications, particularly regarding surface modification and the synthesis of graft copolymers bearing siloxane or polysiloxane segments. The key strategies for both functionalisation and synthesis of siloxane-bearing polymers are highlighted, and the various trends in the development of siloxane-based materials and the intended directions of their applications are explored.
The Si-O bond is a highly versatile chemical linkage that can be found in a great variety of materials and molecules, linking together inorganic [1][2][3] and organic species [4][5][6], as well as being a building block for polymers [7][8][9] and sophisticated 3D oligomers [10][11]. In the role of linkage, the siloxane bond is robust, chemically resistant to an array of environments and easily established, e.g., via the reaction of silanes with organic or inorganic hydroxyl groups [6][12][13]. Conversely, systems containing repeating siloxane bonds—polysiloxanes—tend to exhibit good mechanical properties (ranging from elastomeric to more rigid, dependent on the molecular weight of the polymers, introduced substituents and the use of cross-linking reactions), medium-high solubility in common organic solvents, self-assembling and film-forming properties, as well as low dielectric constants and, frequently, biocompatibility [14].
Consequently, siloxanes have found application for the modification of various surfaces [2][15][16], bestowing upon them a variety of properties, such as increased hydrophobic nature [17]. Conversely, oligo- and polysiloxanes are more commonly employed as primary or auxiliary materials in optoelectronics [18], for biomedical purposes [19] and as protective coatings [20].
In recent years, a multitude of works dedicated to the synthesis and application of both siloxanes and polysiloxanes have been published. Despite this, only a fraction of the reports shows significant progress, whether in terms of scientific novelty or material properties. Consequently, this review is dedicated to the most recent highlights (published since the beginning of 2017) in the field of siloxane-based materials, with mentions of individual works being categorised by the type and nature of the siloxane system being reported.
The Si-O bond is the linkage that is most commonly encountered in nature between silicon and a heteroatom. This is due to the strong nature of this bond, resulting in, e.g., Si-C bonds being replaced by Si-O bonds and, eventually, the formation of oligomeric siloxane structures. Such structures show higher flexibility and are more inert chemically in comparison with analogous systems, based on chains constituted by C-C bonds. These properties, among others, are the reason for the significant research interest in polysiloxanes and other organosilicon compounds. Below, we provide a brief overview of the most common synthetic methods for producing such compounds (Figure 1).
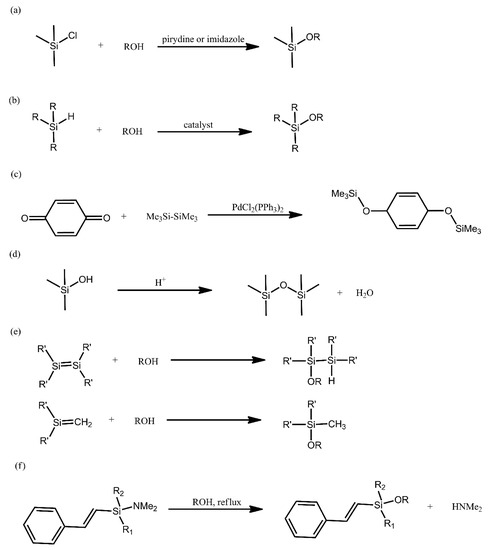
Figure 1. Overview of the most common synthetic methods for producing siloxanes.
Alkoxysilanes are widely used in chemistry, since they can act as linkers between both organic and inorganic species, forming hybrid compounds.
The most common method for producing alkoxysilanes is the condensation of chlorosilanes with alcohols, being typically performed in alkaline environments and in the presence of such bases as pyridine, imidazole and tertiary amines (Figure 1a). A significant drawback of this reaction is the need to employ acid scavenger chemicals, in order to remove the evolving acid from the reaction environment. This is due to the fact that the acid can promote Si-O bond cleavage [21].
Alcoholysis of hydrosilanes is a promising alternative to the above method. The advantage of this reaction is that hydrogen is its only by-product (Figure 1b). The key disadvantage of this approach is the need to employ a catalyst under ambient conditions, because alcohols are not sufficiently nucleophilic. Consequently, research on finding suitable catalysts for this reaction has been on-going for many years, yielding a variety of results, each with their own drawbacks and advantages. This has taken us from catalysts that required elevated temperature, through catalysts that promoted the formation of harmful by-products and onwards. One of the most recent works devoted to that aspect deals with a system composed of 2,2,2-trifluoroacetophenone in the presence of hydrogen peroxide, which was used to obtain silanols with high yields [22].
Alkoxysilanes can also be obtained with the use of dihydroxyaromatic compounds, via the reducing disililation of quinones, with Pd- and Rh-based catalysts being typically used in this reaction (Figure 1c). Many recent works on the synthesis of alkoxysilane oligomers have turned to using this method.
In order to induce the formation of a Si-O bond, acid-catalysed condensation of silanols can also be conducted (Figure 1d). This reaction is frequently employed for obtaining linear siloxanes, silsesquioxanes, silica networks and glasses. The use of strong acids, however, leads to the equilibrium of the reaction being rapidly achieved.
Unsaturated silicon compounds are highly reactive and can yield alkoxysilanes when treated with alcohols (Figure 1e). Due the lack of a catalyst, the nucleophilic addition of water and alcohols to disilenes requires elevated temperatures and takes place at a significantly slower rate than the nucleophilic addition for silenes.
Due to the unstable nature of Si-N bonds, they can be readily converted into Si-O bonds and such a reaction is employed, e.g., for producing sillyl ethers attached to phenol moieties (Figure 1f) [21].
Mesoporous carbon, which has been treated to possess carboxyl groups on its surface, is reported to be readily functionalised with aminopropyl-terminated poly (dimethylsiloxane) (PDMS) [23] via the formation of carboxylates and their conversion to amides. The aim of such a functionalisation was to improve both the electrical properties and cesium ion sorption capacity of the carbon. Functionalisation appears to take place uniformly on the surface of the carbon and largely preserves the morphology of the treated carbon. In terms of electrical properties, functionalisation leads to a 3-5-fold increase in conductivity, depending on the method used for treating carbon (0.22 mS/cm for the untreated carbon; 0.63 and 1.1 mS/cm for functionalized carbon treated by peroxide and plasma respectively) to produce carboxyl groups on its surface. In terms of cesium uptake, the treated carbon (both by peroxide and by plasma) shows a capacity of 0.1 mg cesium cations per gram of carbon, whereas the functionalized carbon shows capacities of 48.1 and 25.9 mg/g for peroxide and plasma-treated carbon respectively, showing a many fold improvement, making the material a potential solution for cesium removal applications.
An interesting approach to functionalising silica surfaces with polysiloxane grafts is to employ a siloxane bond-breaking reagent (dimethyl carbonate or diethyl carbonate) on a mixture of a poly (organosiloxane) and nanosilica particles [24]. These species were then investigated in detail by 1H, 13C and 29Si NMR spectroscopy, with all the observed signals being assigned to particular chemical species grafted onto the surface of the silica nanoparticles.
A continuation of this work, limited to the use of dimethyl carbonate and PDMS to functionalise silica nanoparticles, was carried out, focusing on the adsorption of PDMS oil on the modified nanoparticles and on the molecular dynamics of the obtained systems [25]. PDMS with various molecular weights were used (4–40·kg/mol), yielding a total coverage of the silica grain surfaces in the case of low-MW PDMS and a lower coverage (≈60%) for high-MW (molecular weight) PDMS. Although there is no mention of the amount or mass of grafted PDMS or the siloxane bond-breaking catalyst, such a trend would be easily predicted, assuming constant amounts of PDMS and dimethyl carbonate are used, regardless of the MW of the employed PDMS. This is because for high-MW PDMS the chain count in a unit mass of the polymer will be significantly lower than for low-MW PDMS. Even though dimethyl carbonate, which is consumed upon reaction with a siloxane bond, would increase the chain counts, the absolute chain count increases would be the same, regardless of the MW of PDMS.
The surface of urea-formaldehyde foams was functionalised with polysiloxane grafts in order to make it more hydrophobic [5]. First, the foams were treated with 1,6-hexanediol diglycidyl ether, utilising the reaction of its epoxy functionalities with the amine functionalities present on the surface of those foams. Subsequently, the foams were treated with aminopropyl-terminated PDMS, which reacted with the remaining epoxy functionalities of the grafted 1,6-hexanediol diglycidyl ether molecules (Figure 2). The modified foams were found to indeed be more hydrophobic than the unmodified foams, with their contact angle values for water being reported as 143.4° and 123.6°, respectively.
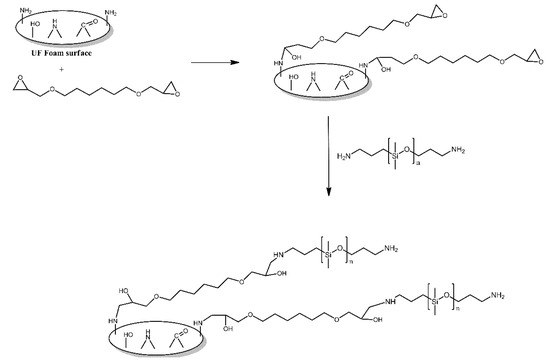
Figure 2. Schematic illustration of urea-formaldehyde foam surface grafted with aminopropyl-terminated PDMS via the bridging effect of 1, 6-hexanediol diglycidyl ether. Based on Ref. [5].
Zang proposed a method for obtaining reversible, temperature-induced colour changing cotton fabric by grafting to cotton fabric via reacting with epoxy modified thermochromic capsule. Epoxy modified thermochromic capsule was prepared by the hydrolysis-polycondensation of siloxane groups of 3-glycidoxypropyltrimethoxysilane with the hydroxy groups of the capsules. The epoxy groups on the surface of thermochromic capsules reacted with the hydroxy groups of cotton fabric and formed covalent bonds (Figure 3) [4]. The modified cotton fabrics changed reversibly from blue to white, the same as the capsules that were not bonded to the fabric. The covalent bonding between the fabric and the capsules resulted in excellent washing and rubbing resistance.
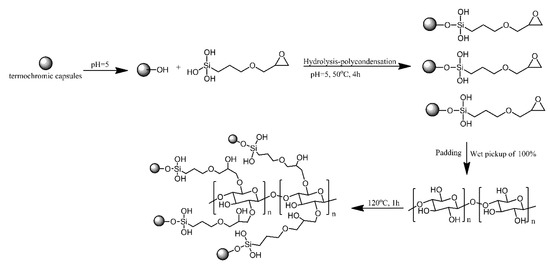
Figure 3. Schematic of thermochromic functionalized cotton fabric with epoxy modified thermochromic capsule. Based on Ref. [4].
An interesting approach to the functionalisation of alloys is reported for a HfNbTaTiZr alloy [12]. The surface of this alloy was first oxidised, so as to functionalise it with hydroxyl groups, followed by treatment with oligo (ethylene glycol) or alkylamino-equipped trimethoxysilanes in ethanol. By reacting with the surface hydroxyl groups, the silanes were transformed into siloxanes and bound to the surface of the alloy. This surface modification method was found to prevent metal release from the surface of the alloy, when immersed in phosphate-buffered saline for an extended period, a desirable feature for potential bioimplant applications.
Epoxy-equipped polyhedral oligomeric silsesquioxanes (POSS) were grafted onto the surface of carbon fibres as part of the procedure for producing carbon fibre-reinforced epoxy composites [16] for potential applications in low earth orbit environments. The carbon fibres were first subjected to several reactions, in order to functionalise them with amino groups. These groups were then reacted with the epoxy functionalities of the POSS molecules. The modified carbon fibres were then treated with an epoxy resin and curing agent, in order to produce the final composites. The resultant composite exhibited improved adhesion between the epoxy phase and the carbon fibre reinforcement, possibly due to curing taking place between the POSS epoxy functionalities and the epoxy resin, as well as improved resistance to atomic oxygen erosion and increased interlaminar shear strength. The composites were prepared by reacting amine groups on the pre-treated carbon fibre surface with the POSS to form a continuous uniform layer of siloxane oligomers. X-Ray photoelectron spectroscopy, scanning electron microscopy and infrared (IR) spectroscopy demonstrated that POSS was successfully grafted onto the carbon fibres surface.
An interesting method for obtaining dendrite-free lithium batteries was proposed by Meng et al. [3]. Utilizing the ability to attach siloxanes to hydroxyl groups on the lithium metal surface with the formation of a siloxane metal bond. Lithium as the active metal has hydroxyl groups on its surface, so the authors apparently used them to react with a liquid, methoxy-terminated PDMS, producing a layer on the lithium metal surface, in order to achieve deposition of uniform lithium layers for batteries.
A triethoxysilane-functionalised tetrazine derivative was grafted onto the surface of indium-tin oxide (ITO) electrodes via immersing the electrodes in a solution of this derivative that also contained acetic acid (Figure 4) [1]. Although no mechanism is mentioned, the authors have likely relied on the presence of hydroxyl groups on the ITO surface and their reaction with the silane-functionalised tetrazine to form siloxane bonds for modifying the electrodes. The tetrazine-modified electrodes were found to exhibit notable fluorescence, which was dependent on the oxidation state of the tetrazine moiety, making it an interesting material for potential sensing or display applications.
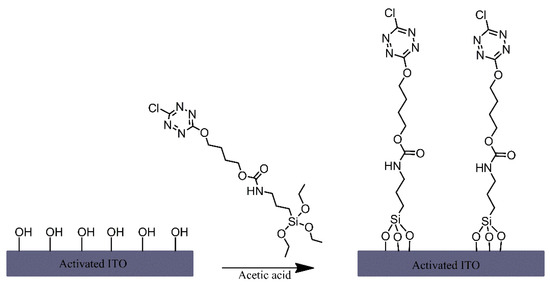
Figure 4. Formation of tetrazine terminated alkyl chain monolayers on indium-tin oxide (ITO). Based on Ref. [1].
Glass, ITO and Si (100) wafer surfaces were modified with Ru2+ terpyridyl complexes, in order to produce materials for Hg2+ sensing [13]. First, the substrate surfaces were treated with an iodo-functionalised trimethoxysilane, utilising its reaction with the hydroxyl groups present on those surfaces. Subsequently, the iodo-functionalised surfaces were treated with one of the three terpyridyl derivatives, via a SN2 coupling reaction between the iodo-functionalised alkyl chains and pendant pyridyl groups or primary amino group of the complexes (Figure 5). The optical and electrochemical properties of the respective functional monolayers were studied in detail and their potential for detecting ppm levels of highly toxic Hg2+ ions was tested.
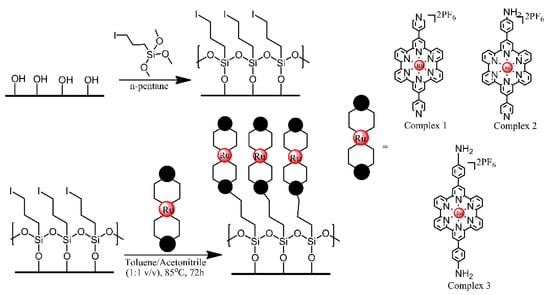
Figure 5. Schematic Representation of the Two-Step Fabrication Process for the Covalent Assembly of Functional Ru(II) Complexes 1−3 on Freshly Activated, Hydroxylated SiOx-Based Substrates (Si/Glass/ITO), Resulting in the Formation of metalloligand monolayers. Based on Ref. [13].
Titanium substrates were modified using imidazolium-terminated trialkoxysilanes, in order to produce surfaces resistant to bacterial colonization [2]. The titanium substrates were first treated, cleaned with ethanol and toluene, and then oxidised under acidic conditions. Next, instead of directly reacting the alkoxysilanes with hydroxyl groups present on the surface of the substrates, the alkoxysilanes were irradiated, resulting in cross-linking between neighbouring silane molecules and introduction of hydroperoxide substituents to the silicon atoms. These hydroperoxide functionalities were then used to anchor the silanes onto the substrates via the hydroxyl groups on their surface (Figure 6). The modification of the substrates resulted in a significant improvement in terms of the resistance of the surfaces to bacterial colonisation by E. coli and S. aureus. Unfortunately, this resistance was found to be short-term, as it was lost after approximately 24 h.
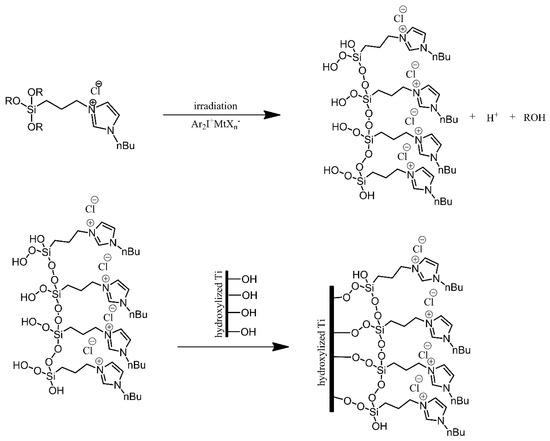
Figure 6. Photografting of the imidazolium-derived siloxane on a titanium plate. Based on Ref. [2].
Silica aerogel surfaces were functionalised with modified aramid fibres, in order to produce materials with lower thermal conductivity [15]. Aramid fibres were first nitrated, introducing nitro groups into their aromatic rings. These nitro groups were then reduced to amino groups, which in turn were treated with epoxy-functionalised silanes. Similarly to other works mentioned here, the silanes were used to couple with the hydroxyl groups present on the silica aerogel surfaces and anchor the grafts to them (Figure 7). Only a small decrease in thermal conductivity was observed (thermal conductivity coefficient of 0.032 W/m·K for the silica-modified fibres, in comparison for 0.042 W/m·K for the non-modified aramid fibres).
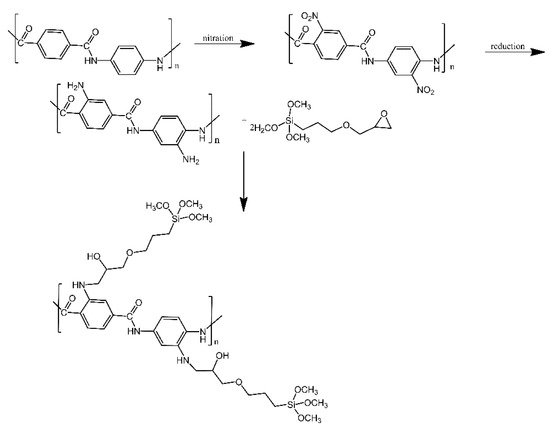
Figure 7. Mechanism for preparation of g-(amino groups aramid fibre). Based on Ref. [15].
Two types of pyridinium N-chloramine precursors were anchored to the surface of cotton utilising the reaction of their alkoxysilane functionalities with the hydroxyl groups present on the surface of cotton (Figure 8) [6]. Following grafting, the precursors needed to be chlorinated with bleach, in order to temporarily bestow upon them antibacterial properties. The treated and activated surfaces were found to be effective against E. coli and S. aureus, although there is no mention of the duration for which these properties persisted.
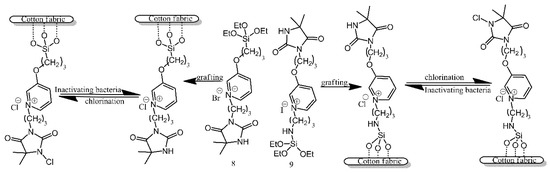
Figure 8. Schematic illustration of cotton fabric grafting and chlorination. Based on Ref. [6].
An interesting approach to producing non-fouling and antibacterial multifunctional surfaces was reported by Wang et al. First, PDMS was treated with an amino-functionalised silane, in order to produce amine groups on the surface of PDMS. Next, those amino groups were utilised in a reversible addition-fragmentation chain transfer (RAFT)-type polymerisation reaction, to produce methacrylate copolymer chains that contained phosphorylcholine pendant groups (Figure 9). Lastly, the methacrylate copolymer chains were treated with bromoheptane, in order to convert their amine functionalities into quaternary ammonium moieties. The modified surfaces showed efficiency in reducing bovine serum albumin adsorption, inhibiting bacteria adhesion and biofilm formation, as well as bactericidal properties towards S. aureus [26].
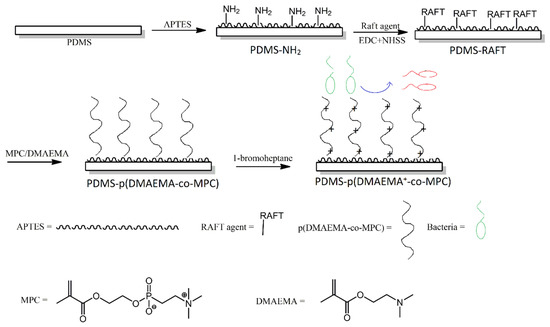
Figure 9. Schematic illustration of the preparation of p(DMAEMA+-co-MPc) copolymer brushes on poly (dimethylsiloxane) (PDMS) with nonfouling and bactericidal properties. Abbreviations: p (DMAEMA+-co-MPc) = (2-(dimethylamino)-ethyl methacrylate-co-2-methacryloyloxyethyl phosphorylcholine; APTES = (3-aminopropyl)triethoxysilane; EDC = N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride; NHSS = N-hydroxysulfosuccinimide sodium salt. Based on Ref. [26].