1000/1000
Hot
Most Recent

Smoking may increase the risk of various neuropsychiatric diseases, such as dementia/cognitive decline, schizophrenia/psychosis, depression, anxiety disorder, and suicidal behavior induced by structural and functional alterations of the central nervous system, mainly centered on inflammatory and oxidative stress pathways.
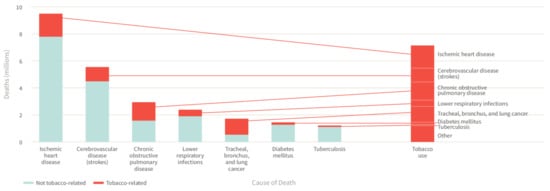
The tobacco epidemic represents one of the biggest global public health challenges, remaining a leading cause of global morbidity and mortality, despite substantial efforts to combat tobacco use such as the MPOWER measures introduced by the World Health Organization (WHO) in 2008 [1]. The WHO estimates that tobacco use is responsible for more than 8 million global deaths each year, killing up to half of its users and thus making it the single greatest preventable cause of death [2]. While cigarette smoking is a well-established risk factor for non-communicable diseases and related deaths including cardiovascular disease, cancer, chronic lung disease, and diabetes mellitus ( Figure 1 ) [3], much less is known about its impact on neuropsychiatric disease risk as well as the underlying pathomechanisms. Since neuropsychiatric diseases constitute a major contributor the global burden of disease and public health costs, there is an urgent need to identify relevant risk factors and constellations, making smoking a potential target of neuropsychiatric disease prevention strategies. In the present review, we provide an updated overview of the effects of smoking on neuropsychiatric disease risk as well as other main diseases, along with pathophysiological insights from human and animal studies centered on inflammatory and oxidative stress pathways.
As far back as 1939, it was noticed that smoking was associated with an increase in lung cancer incidence [4]. It was only in the 1960s, after large epidemiological trials and novel mechanistic insight had emerged, that the general public and the medical and scientific community officially acknowledged the causal link between tobacco smoking and lung cancer [5][6]. Now that the causal link between tobacco smoking and lung cancer is evident, other types of cancer are being not only associated, but also causally linked to tobacco smoking. Breast cancer, the most common type of cancer, was found to be correlated with smoking. A report from the California Environmental Protection Agency from 2005 was the first report of a major health institution, stating that contemporary data provided support for a causal association between current smoking and elevated breast cancer risk [7]. In the following years, other institutions have provided reports, indicating at least a correlation between tobacco smoking and incidence of breast cancer [8][9]. A recent meta-analysis found that women who smoked during their life had an overall relative risk (RR) of 1.10 in prospective studies and 1.08 in retrospective studies of developing breast cancer [10]. The overall RR increased to 1.13 for current smokers in prospective studies.
Prostate cancer, the third most common type of cancer, is not directly correlated with tobacco smoking [11], but tobacco smokers have poorer survival and recurrence-free rates after prostate cancer diagnosis [12]. Bladder cancer, on the other hand, is known to be caused by tobacco smoking [11]. In a meta-analysis including 107 studies on bladder cancer, the RR for all smokers was 2.58 and even reached 3.47 for current smokers [13].
Other types of cancer were also correlated with tobacco smoking. Increased risk of cervical cancer was correlated with smoking, with an odds ratio (OR) of 2.17 for human papillomavirus positive women [14]. The risk of developing cervical cancer was also found to be elevated in passive smokers, indicating that even women who do not actively smoke are at risk [15]. Pancreatic cancer was also observed to be correlated with tobacco smoking. A recent meta-analysis reported an RR of 1.66 when compared to never smokers [16]. As with prostate cancer, the survival after a pancreatic cancer diagnosis was reduced with a hazard ratio (HR) of 1.37 compared to never smokers and the HR increased with the amount of tobacco consumed (pack-years) [17]. Tobacco smoking is also found to be linked with oral cancers [18], head and neck cancers [19], and ovarian cancer [20].
Studies from the 1960s recognized that smoking causes not only lung cancer, but other types of respiratory disease such as chronic bronchitis [6]. Chronic obstructive pulmonary disease (COPD) is one of the most common lung diseases associated with tobacco smoking. It is now accepted that tobacco smokers have a much higher risk of developing COPD and that almost 50% of all smokers are expected to develop COPD during their life [21]. Smoking cessation after COPD diagnosis is the major factor for reducing mortality [22]. Smoking was also associated with higher incidence of adult asthma in Finland, with an OR of 1.33 [23]. Globally, it is not clear if there is a causal link between smoking and asthma, but it is clear that smoking in asthmatic populations leads to higher risk of developing more severe pulmonary disease like COPD [24]. Interstitial lung diseases that result in damage of lung interstitial tissue were shown to be smoking-dependent [25]. Pulmonary Langerhans cell histiocytosis is an example of a pulmonary disease that is almost completely dependent on smoking, as smoking is considered a major, and maybe the only, risk factor [26]. Respiratory bronchiolitis is an inflammatory response to chronic tobacco smoke injury and is only curable by smoking cessation [27]. From all of the cases of adult desquamative interstitial pneumonia, over 90% are smokers [28]. Smoking is also a recognized risk factor for idiopathic pulmonary fibrosis, however has not been causally linked [29].
The ability of tobacco smoke to cause oxidative stress and systemic inflammation has far-reaching consequences. Besides causing cancer, lung disease, and cardiovascular disease, tobacco smoking is associated with different neuropsychiatric diseases.
Lower activity of the antioxidant enzyme paraoxonase was associated with major depression disorder [30]. The same study found that smoking reduces the activity of paraoxonase, which could classify smoking as a risk factor for major depression disorder acting through oxidative stress. Presence of 8-hydroxy-2-deoxyguanosine, a marker of DNA damage by free radicals, was found in the urine and plasma of patients suffering from depression [31]. Increased NO protein modifications were observed in the serum of depressed patients, further strengthening the link between nitrosative stress and depression [32]. Parental smoking is also a risk factor for developing autism spectrum disorder in the offspring, based on the induced oxidative stress [33]. Both oxidative stress and inflammation were increased in the brains of post-traumatic stress disorder model animals [34]. Oxidative stress markers are prominent features of neurodegenerative diseases and neuropsychiatric disorders. Oxidative stress leads to lipid peroxidation and the creation of products such as 4-hydroxynonenal. 4-hydroxynonenal can induce inflammation and apoptosis of neuronal cells, lead to accumulation of peroxides in astrocytes [35], impairment in axon regeneration, aberrations in axonal functioning, loss of active mitochondria, and suppression of mitochondrial respiration [36][37]. All of these detrimental effects of oxidative stress have a high impact on neurodegenerative diseases like Parkinson’s disease and Alzheimer’s disease. Loss of motor function in Parkinson’s disease caused by degradation of dopaminergic neurons is promoted by an accumulation of alpha-synuclein. Accumulation of alpha-synuclein is both increased and increases oxidative stress itself, producing a neurodegeneration cycle [38]. Compelling data demonstrates that impaired neuronal metal homeostasis could be involved in the formation of oxidative stress, influencing amyloid aggregation in case of Alzheimer’s disease [39].
High levels of cytokines have been shown to associate with anxiety and depressive mood [40], leading to a hypothesis that chronic inflammation could be responsible for these psychiatric diseases [41]. Higher levels of circulating cytokines, mostly IL-6 and tumor necrosis factor alpha (TNFα), were found in patients with schizophrenia and bipolar disorder [42]. Microglia activation observed through translocator protein binding, a measurement of neuroinflammation, demonstrated that inflammation is implicated in obsessive-compulsive disorder [43]. Attention-deficit/hyperactivity disorder (ADHD) is a neuropsychiatric disease that affects children, but it was observed that parental smoking was a risk factor for developing ADHD [44]. This association was made through the presence of pro-inflammatory cytokines in ADHD children whose fathers were smokers [45]. Same as with oxidative stress, inflammation is implicated in neurodegenerative disease as well, leading to neuropsychiatric features. Both Parkinson’s and Alzheimer’s disease are accompanied by local and systemic inflammation [46]. The relationship between smoking, oxidative stress, inflammation, and neuropsychiatric disease is not always clear. This stems from the fact that many neuropsychiatric diseases also increase the chance that a person will start smoking [47], making the direction of association difficult to establish ( Figure 2 ).
Tobacco smoking is not only implicated in neuropsychiatric disease through oxidative stress and inflammation, but also through direct exposure to some of the chemicals present in tobacco smoke. High environmental concentrations of lead have caused its accumulation in plants and the tobacco plant is no exception. Lead is known to induce schizophrenia [49] and increased intake of lead from tobacco is a risk factor for developing schizophrenia later in life. Nicotine, a highly addictive substance that modifies neurotransmitter patterns, can have a direct influence on neuropsychiatric diseases like schizophrenia and depression [50]. Interestingly, there is also evidence for procognitive effects of nicotine in subjects with neuropsychiatric diseases such as schizophrenia [51][52] and ADHD [53][54], which may explain sustained smoking in these disease phenotypes. In addition, chemicals present in smoke may interact with antipsychotics, antidepressants, and benzodiazepines through pharmacokinetic and pharmacodynamic (mainly nicotine-mediated) pathways [55], showing that, for example, smoking cessation in patients receiving clozapine may lead to elevated plasma concentrations of clozapine and severe side effects via altered metabolic clearance by CYP1A2 [56]. More details on the contribution of nicotine to neuropsychiatric disorders were reviewed in references [50][57][58]. Lastly, it is highly important to note that (neuro)psychiatric disorders have a strong link with chronic stress, which represents one of the more prominent risk factors for their onset. In this context, cancer, cardiovascular, and metabolic disorders, together with many other chronic pathologies, represent severe forms of chronic stress, intuitively increasing the risk of neuropsychiatric disorders.
Although there is ample evidence indicating an interrelationship between smoking and neuropsychiatric diseases, meaning that rates of smoking are markedly higher in subjects with prevalent neuropsychiatric disease than in the general population being two to five times higher including subjects with, for example, schizophrenia, depression, anxiety disorders, ADHD, binge eating disorders, bulimia, and substance use disorders [59] ( Figure 2 ), much less is known about the prospective impact of smoking on neuropsychiatric disease development. However, emerging strong evidence from epidemiological studies suggests that smoking may be a causal factor for the development or progression of neuropsychiatric disease.
In the Gutenberg Health Study, a prospective cohort study from Germany, Beutel et al. evaluated longitudinal data of 10,036 participants, demonstrating that current smoking was predictive of new onset of depression, with an OR of 1.35 (95% confidence interval (CI) 1.05–1.71), which remained stable after further adjustment for subclinical depression at baseline [60]. Cabello et al. analyzed data from 7908 participants from Ghana, India, Mexico, and Russia from the WHO’s Study on Global Ageing and Adult Health, showing that current daily (OR 1.46, 95% CI 1.09–1.97) as well as non-daily (OR 2.06, 95% CI 1.18–3.62) smoking was associated with incident depression [61]. In the Copenhagen City Heart Study (N = 18,146), smoking more than 20 g of tobacco per day was independently associated with incident depression in women (HR 2.17, 95% CI 1.45–3.26) and men (HR 1.90, 95% CI 1.05–3.44) after a follow-up of up to 26 years [62]. A Norwegian study (N = 1190) analyzed the association between smoking and subsequent first depression [63]. The authors demonstrated that HRs for incident depression followed a dose-dependent association for former and current smoking, with current smokers who smoked more than 20 cigarettes per day having the highest risk of developing depression (HR 4.34, 95% CI 1.85–10.18). In an Australian case-control study, smoking was cross-sectionally (N = 165 cases and 806 controls, age-adjusted OR for smoking more than 20 cigarettes per day 2.18, 95% CI 1.31–3.65) and prospectively (N = 671, HR 1.93, 95% CI 1.02–3.69, not explained by physical activity or alcohol consumption) associated with increased risk of major depressive disorder in women [64]. Interestingly, on the basis of the National Longitudinal Study of Adolescent Health from the United States (US), Goodman and Capitman revealed that current smoking in adolescents (N = 8704) was strongly predictive of developing high depressive symptoms, whereas in non-current smoking adolescents (N = 6947) high depressive symptoms were not predictive of heavy smoking after multivariable adjustment, challenging the common assumption of causality in the context of smoking and depression that rather goes in the direction from depression to smoking [65]. In good agreement, in the Korea Welfare Panel, smoking was shown to predict depression, whereas no association was found when testing for the opposite direction for the relationship of depression and smoking [66]. Conversely, Munafò et al. showed within the same cohort that baseline smoking status did not predict depressed mood at follow-up in adolescents, although there was a trend towards a significant relationship in females [67]. A more recent systematic review on the association between smoking, depression, and anxiety including a total of 148 studies found that smoking status was positively associated with later depression in most studies (37 out of 51 studies), while relatively few studies (14 out of 51 studies) found no evidence for this relationship [68]. Evidence for a bidirectional relationship between smoking and poor mental health/depressive mood arises from a longitudinal analysis of young Australian women (N = 10,012), indicating that smoking was associated with higher odds of depressive mood at subsequent waves, with smoking more than 20 cigarettes per day displaying the highest odds [69]. Conversely, women with poor mental health/depressive mood had higher odds of smoking at subsequent waves. Khaled et al. analyzed data from the Canadian National Population Health Survey, demonstrating that heavy smoking was associated with onset of major depression (HR 3.1, 95% CI 1.9–5.2) even after adjustment for mental stress [70].
In the Nurses’ Health Study II (N = 116,363 women), risk of incident seizure or epilepsy in response to smoking, caffeine use, and alcohol intake was examined [71]. Only smoking was shown to be associated with increased risk of incident events, with current smoking displaying an RR of 2.60 (95% CI 1.53–4.42) for seizure and an RR of 1.46 (95% CI 1.01–2.12) for epilepsy in response to former smoking.
A meta-analysis of 15 studies on maternal smoking during pregnancy and risk of autism spectrum disorder in offspring found no evidence for an association (OR 1.02, 95% CI 0.93–1.12) [72]. Comparable results were achieved by a further meta-analysis including 22 studies (OR 1.16, 95% CI 0.97–1.40), while a substantial relationship was revealed after considering population-level smoking metrics (i.e., adult male smoking prevalence) [73]. In support of this, maternal smoking during the whole pregnancy was associated with an increased risk of pervasive developmental disorder in the offspring in a Finnish cohort study (OR 1.2, 95% CI 1.0–1.5), which remained stable after further adjustment for maternal age, mothers socioeconomic and psychiatric status, and infants weight for gestational age [74]. In contrast, Caramaschi et al. found no compelling evidence for a causal association between maternal smoking during pregnancy and offspring autism or related traits using Mendelian randomization and a genetic variant at the CHRNA3 locus in maternal DNA as a proxy for heaviness of smoking [75].