Despite the progressive advances, current standards of treatments for peripheral nerve injury do not guarantee complete recovery. Thus, alternative therapeutic interventions should be considered. Complementary and alternative medicines (CAMs) are widely explored for their therapeutic value, but their potential use in peripheral nerve regeneration is underappreciated. Various CAMs enhanced proliferation and migration of Schwann cells in vitro, primarily through activation of MAPK pathway and FGF-2 signaling, respectively. Animal studies demonstrated the ability of CAMs to promote peripheral nerve regeneration and functional recovery, which are partially associated with modulations of neurotrophic factors, pro-inflammatory cytokines, and anti-apoptotic signaling.
1. Introduction
Peripheral nerve injury (PNI) can result in partial or total loss of motor, sensory and autonomic functions at denervated regions, leading to temporary or life-long disability
[1]. In addition to reduced quality of life, functional deficits from PNI have a substantial economic impact on the affected individuals
[2]. A recent study found that, over nine years (from 2009 to 2018), more than 550,000 individuals were afflicted by PNI in the United States. Moreover, the incidence rate has more than doubled throughout that period of time
[3]. Such injuries are primarily due to vehicular and traumatic accidents, lacerations, and iatrogenic causes
[4][5][6].
Despite progressive advances in our understanding of the processes and mechanisms of nerve injury, effective nerve repair and regeneration approaches that ensure complete functional recovery remain scarce
[7]. Nerve autograft is considered the gold standard for repairing peripheral nerve defects
[8]. However, this method is restricted by limited donor nerves and donor site morbidity, while successful recovery rates remain unsatisfactory
[9]. Consequently, alternative strategies for enhancing nerve repairs have been proposed, including the application of nerve conduits and the addition of growth factors
[10][11]. Likewise, the exploration of novel therapeutics, even combinatorial therapies, capable of enhancing axonal regeneration and promoting functional recovery, are of great interest.
PNI often results in neuropathic pain, and when conventional treatments are inadequate in providing relief, patients may turn to complementary and alternative medicines (CAMs), such as herbal medicines and nutritional supplements
[12]. Indeed, medicinal plants, including the
Acorus calamus [13],
Curcuma longa [14], and
Ginkgo biloba [15], have displayed ameliorating effects in animal models of neuropathic pain. Research on the potential of medicinal plants in the treatment of PNI is prompted by the notion that plants are great sources of natural products (NPs), which are small molecules produced by living organisms. Many NPs are the focus of drug development, as it is generally believed that they are largely devoid of adverse effects compared to synthetic drugs
[16][17]. NPs also have the advantage of being evolutionary-driven, thus they are more likely to possess tremendous chemical and structural diversity that facilitates efficient engagement with biologically relevant targets and receptors, making them more biologically active
[18]. In fact, many small-molecule drugs that have been approved by regulatory agencies were derived from natural sources
[19], including Taxol from
Taxus brevifolia [20] and Vinblastine from
Catharanthus roseus [21].
However, compared to the extensive research on naturally derived products for other non-communicable and infectious diseases, NPs remain largely unexplored in the field of nerve repair and regeneration.
2. Complementary and alternative medicines (CAMs) and Peripheral Nerve Regeneration
2.1. Current Therapeutic Approaches against Peripheral Nerve Injuries
Peripheral nerves are prone to injury because of their delicate structures and superficial location throughout the human body. The prevalence of PNI together with its societal impact poses a health concern that needs to be addressed properly. Current treatment strategies for PNI are divided into surgical and non-surgical approaches that can be effective when applied appropriately
[22]. Surgical techniques, including suturing of severed nerves and nerve grafting, do yield successful outcomes but are sometimes not feasible due to limitations such as the timing of surgery, size of nerve gaps, and donor site morbidity
[23][24]. Consequently, other promising alternatives have emerged in recent years and have been receiving increasing attention, such as the utilization of different nerve conduits capable of housing and delivering biological cues whilst enhancing and guiding nerve regeneration 11, growth factor treatments
[25], and cell-based therapies
[26]. In contrast, non-surgical options for the management of PNI are far more limited, including approved medications on the market, electrical nerve stimulation
[27], and the application of phytochemicals and secondary metabolites. The latter is widespread in other areas of research including cancer
[28] and neurological disorders
[29], but are far less prevalent in the field of peripheral nerve regeneration.
2.2. Mechanisms of Peripheral Nerve Injury and Regeneration
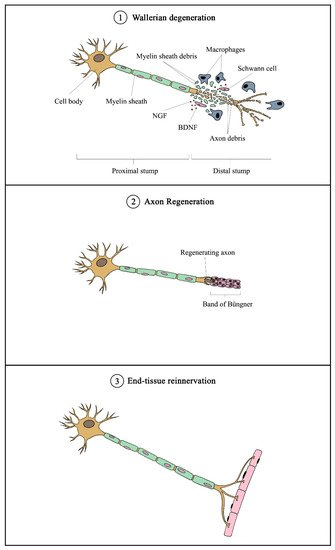
Nerve bundles are primarily composed of axons covered with myelin sheaths produced by Schwann cells with fibroblasts scattered in between the nerve fibers. During peripheral nerve injury, instantaneous tissue damage occurs at the site of the lesion together with the accumulation of galectin-3 macrophages, whereas nerve stumps that are distally located undergo cellular variation despite not being directly affected
[30]. After an axonal injury, Wallerian degeneration occurs, followed by axonal regeneration, and eventually end-organ reinnervation (see
Figure 1)
[31]. Wallerian degeneration takes place 24 to 48 h following nerve injury. Axons begin to disintegrate and growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are released by SCs in the segment distal to the injured site. Galectin-3 macrophages are then recruited to the distal end, which contributes to myelin degradation and removal of remaining debris
[32]. Growth factors are also retrogradely transported proximally toward the cell body. Subsequent removal of deteriorated myelin and axonal matter leads to the proliferation and alignment of SCs, forming the bands of Büngner that further guide the regenerating axons from the proximal to the distal site
[33]. Axonal regeneration in humans is known to occur at a rate of approximately 1 mm per day
[34], meaning that it would require months or even years for severe nerve injuries to fully recover. Moreover, poor functional recovery can occur due to a number of reasons, including progressive failure of axonal regeneration, disruption of SC function in providing a growth-supportive environment, and misdirection of regenerating axons
[34].
Figure 1. Overview of mechanism of peripheral nerve injury and regeneration. Following nerve injury, Wallerian degeneration occurs, in which axons begin to disintegrate at the distal end, and growth factors (such as NGF and BDNF) are released by Schwann cells. Galectin-3 macrophages are recruited to remove axonal debris and degrade myelin sheaths. Subsequently, SCs align to form the Band of Büngner, which guides the regenerating axons from the proximal to distal sites. Eventually, the regenerated axons innervate the end tissue to complete the recovery process. NGF—nerve growth factor; BDNF—brain-derived neurotrophic factor.
2.3. Role of Schwann Cells in Nerve Regeneration
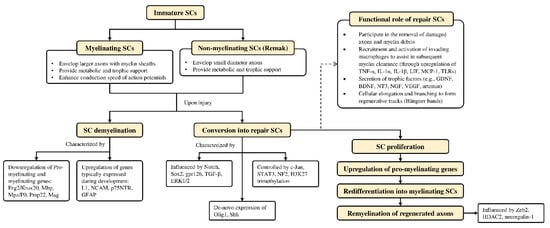
Schwann cells are supportive glial cells that are known to play a pivotal role in the proper functioning and maintenance of peripheral nerves. They are responsible for producing the basal lamina that determines the polarity of SCs and myelinating axons [35]. The myelin sheaths on axons allow the conduction of action potentials at high velocity via the formation of specialized nodes of Ranvier [36]. The high plasticity of SCs allows them to further develop into repair phenotypes in response to nerve injury (Figure 2). Following nerve injury, SCs can re-differentiate into repair SCs that align themselves to form bands of Büngner. This in turn allows axons to emerge from growth cones proximal to the injured site, which then elongate along the bands until the target organ is reinnervated. The repair SCs also participate in the removal of axon and myelin debris, and they can recruit macrophages to assist in the process [37]. In addition, repair SCs can also secrete neurotrophic factors that help promote cellular survival, proliferation, and differentiation, which are all essential for peripheral nerve repair [38]. Due to the importance of SCs in promoting peripheral nerve regeneration, it is expected that any disruption in SC proliferation, such as that caused by impairment in cyclin D1, will affect nerve regeneration following injury [39]. However, findings from past studies suggest that axonal regeneration is independent of SC proliferation [40][41]. Nevertheless, considering the association of SCs with axonal elongation and myelination, it is reasonable to hypothesize that enhanced SC proliferation may lead to greater regenerative potential.
Figure 2. Overview of Schwann cell plasticity and their roles following peripheral nerve injury. Immature SCs develop into either myelinated or non-myelinated forms depending on the type of axon association. Upon nerve injury, SCs are capable of converting into a repair phenotype alongside the demyelination process that is mediated by different genes and transcriptional mechanisms. These events promote neuronal survival and enhance axonal regeneration following injury. Subsequently, repair SCs can be reprogrammed back to remyelinate regenerated axons. Further details on SC plasticity are presented in the reviews by Jessen & Mirsky
[37] and Nocera & Jacob
[42]. BDNF—brain-derived neurotrophic factor; Erg2/Krox20—early growth response 2; ERK—extracellular signal-regulated protein kinase; GDNF—glial cell-derived neurotrophic factor; GFAP—glial fibrillary acidic protein; gpr126—adhesion G protein-coupled receptor G6; H3K27—methylation of histone H3 on lysine 27; HDAC2—histone deacetylase 2; IL—interleukin; L1—L1 cell adhesion molecule; LIF—leukemia inhibitory factor; Mag—myelin associated glycoprotein; Mbp—myelin basic protein; MCP-1—monocyte chemotactic protein 1; Mpz/P0—myelin protein zero; NCAM—neural cell adhesion molecule; NF2—neurofibromatosis 2; NGF—nerve growth factor; NT3—neurotrophin-3; Olig1—oligodendrocyte transcription factor 1; p75NTR—p75 neurotrophin receptor; Pmp22—peripheral myelin protein 22; SCs—Schwann cells; Shh—Sonic Hedgehog; Sox2—(sex determining region Y)-box 2; STAT3—signal transducer and activator of transcription 3; TGF-β—transforming growth factor-β; TLRs—Toll-like receptors; TNF-α—tumor necrosis factor-α; VEGF—vascular endothelial growth factor; Zeb2—zinc finger E-box-binding homeobox 2.
2.4. Experimental Strategies and Neuroprotective Effects of Complementary and Alternative Medicines (CAMs) against Peripheral Nerve Injury
2.4.1. CAMs with Neuroregenerative Potential
Due to the limitations of conventional therapies for PNIs, much attention has been dedicated to finding alternative approaches in treating PNIs. To date, studies have explored the potential of 20 species of plants, three species of mushrooms, and four types of decoctions in promoting peripheral nerve regeneration (Table 1). Notably, the neuroregenerative potential of
Achyranthes bidentata [43][44][45][46][47][48],
Astragalus membranaceus [49][50][51][52],
Curcuma longa [53][54][55][56][57][58],
Panax ginseng [59][60][61], and
Hericium erinaceus [62][63][64] have been most studied. A total of 18 natural products have been identified across the studies. Among those, ursolic acid, syringic acid, and quercetin are the NPs that can be found across a variety of plant species
[65][66][67][68][69][70]. Decoctions are usually made according to traditional formulae. However, among the decoctions discussed in this study, the Bogijetong decoction is a relatively modern formulation that was specifically developed to treat neuropathic pain
[71].
Table 1. Plant and mushroom species, decoctions, as well as associated natural products that were used in studies investigating the effects of CAMs on peripheral nerve regeneration.
| Source |
Natural product |
Reference |
| Achyranthes bidentata |
- |
[43][44][45][46][47][48] |
| Alpinate Oxyphyllae Fructus (Alpinia oxyphylla Miq) |
Protocatechuic acid |
[72][73] |
| Astragalus membranaceus |
Astragaloside IV |
[49][50][51][52] |
| Centella asiatica |
- |
[74] |
| Citrus medica var. sarcodactylis |
- |
[75] |
| Codonopsis pilosula |
- |
[76] |
| Crocus sativus |
Crocin |
[77] |
| Curcuma longa |
Curcumin |
[77][53][54][55][56][57][58] |
| Honeybee |
Propolis |
[58] |
| Dioscoreae rhizoma |
- |
[78] |
| Epimedium |
Icariin |
[79][80] |
Gardenia jasminoides Ellis
|
Genipin |
[81] |
| Gastrodia elata Blume |
Gastrodin |
[82] |
Ginkgo biloba
|
Ginkgo biloba extract (EGb 761) |
[83][84] |
| Ginseng |
Ginsenoside Rg1, ginsenoside Re |
[59][60][61] |
| Green tea |
(-)-Epigallocatechin-3-gallate (EGCG) |
[85][86] |
| Can be found in a variety of plant species |
Syringic acid |
[65] |
| Can be found in a variety of plant species |
Ursolic acid |
[66] |
| Lycium barbarum |
- |
[87] |
| Can be found in a variety of plant species |
Quercetin |
[67] |
| Morus sp. |
- |
[88] |
| Pueraria lobata |
Puerarin |
[89][90] |
| Radix Hedysari |
- |
[91][92] |
| Rhodiola rosea L. |
Salidroside |
[93] |
Scutellaria baicalensis Georgi
|
Baicalin |
[94] |
Trigonella foenum-graecum
|
- |
[95] |
Tripterygium wilfordii Hook. F.
|
Triptolide |
[96] |
Amanita muscaria
|
Muscimol |
[97] |
Hericium erinaceus
|
- |
[62][63][64] |
| Bogijetong |
- |
[71] |
| Buyang Huanwu |
- |
[98] |
| Jiaweibugan |
- |
[99] |
| Qian-Zheng-San |
- |
[100] |
2.4.2. In Vitro Studies on Neuroregenerative Potential of CAMs
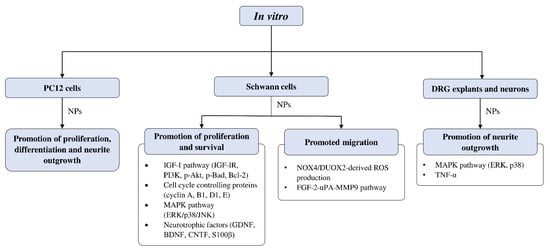
Figure 3 summarizes the
in vitro studies on neuroregenerative properties of complementary and alternative medicines. Most of the studies were in Schwann cells, with a few using DRG explants, neurons, and PC12 cells (rat pheochromocytoma). Some CAMs were reported to induce proliferation, differentiation, and neurite outgrowth in PC12 cells. Similarly, neurite outgrowth was also promoted in DRG neurons through modulation of the extracellular signal-regulated kinase (ERK), p38, and tumor necrosis factor-α (TNF-α). Polypeptides isolated from
Achyranthes bidentata have demonstrated the ability to promote neurite outgrowth in DRG neurons through the activation of ERK1/2
[44][45].
Figure 3. Overview of in vitro studies that demonstrated the effects of natural products relating to peripheral nerve regeneration across different cell types with associated mechanisms. Akt—protein kinase B; Bad—Bcl-2 associated agonist of cell death; Bcl-2—B-cell lymphoma 2; BDNF—brain-derived neurotrophic factor; CNTF—ciliary neurotrophic factor; DRG—dorsal root ganglion; DUOX2—dual oxidase 2; ERK—extracellular signal-regulated kinase; FGF—fibroblast growth factor; GDNF—glial cell-derived neurotrophic factor; IGF-I—insulin-like growth factor 1; IGF-IR—insulin-like growth factor 1 receptor; JNK—c-Jun N-terminal kinase; MAPK—mitogen-activated protein kinase; MMP9—matrix metallopeptidase 9; NOX4—nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4; NPs—natural products; PC12—pheochromocytoma cells; PI3K—phosphoinositide 3-kinase; ROS—reactive oxygen species; S100β—S100 calcium-binding protein β; TNF-α—tumor necrosis factor-α; uPA—urokinase plasminogen activator.
Effects of CAMs on Schwann Cell Activity In Vitro
The studies examining the effects of complementary and alternative medicines and their related natural products on Schwann cells are primarily focused on promoting their proliferation and survival. The molecular mechanisms that were investigated in these studies include signaling pathways such as IGF-I and MAPK, as well as cell cycle controlling proteins and various neurotrophic factors (Figure 3). Past studies have demonstrated that ERK is required for proper myelination of SCs during development [101][102], and ERK signaling was rapidly activated following nerve injury, contributing to SC differentiation [103]. Moreover, evidence suggests that nerve regeneration following injury is closely associated with ERK [104][105], and ERK inhibition leads to impaired regenerative capability [104][106]. On the other hand, inhibition of p38 MAPK prevented SC demyelination and dedifferentiation, indicating its role in promoting the breakdown of myelin following nerve injury [107]. It is not unexpected that cyclins are associated with SC proliferation, as these proteins control cell cycle progression through the interaction of cyclin-dependent kinases. For instance, cyclin D is associated with Cdk4 or Cdk6 in the G1 phase, cyclin A participates with Cdk1 or Cdk2 in the S phase, cyclin E is involved with Cdk2 in G1 and S phases, cyclin B and Cdk1 regulates M phase [108][109].
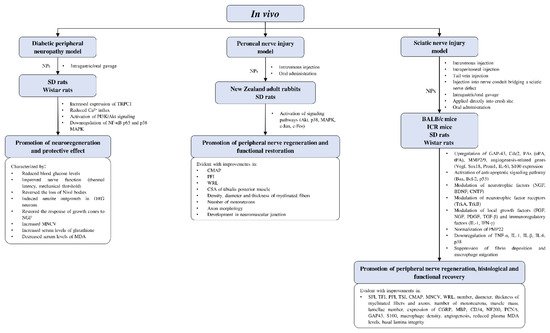
4.4.3. In Vivo Studies on Neuroregenerative Potential of CAMs
Current in vivo studies on the potential of complementary and alternative medicines in peripheral nerve regeneration are limited to rodent models (Figure 4). Most of the studies involved different strains of rats and mice, with only two studies using rabbits as their animal models. Models of peripheral nerve injury used in the studies include diabetic peripheral neuropathy, peroneal nerve injury, and sciatic nerve injury. The effects of CAMs on peripheral nerve regeneration were evaluated by functional recovery indexes (e.g., PFI; sciatic function index, SFI; tibial function index, TFI; CMAP; MNCV; and WRL) and histological examinations (e.g., number, diameter, the thickness of myelinated fibers and regenerated axons; the number of motoneurons; and muscle mass).
Figure 4. Overview of in vivo studies that demonstrated the effects of natural products relating to peripheral nerve regeneration across different experimental models with associated mechanisms. Akt—protein kinase B; Bax—Bcl-2-associated X protein; Bcl-2—B-cell lymphoma 2; BDNF—brain-derived neurotrophic factor; Cdc2—cell division control protein; CGRP—calcitonin gene-related peptide; CMAP—compound muscle action potential; CNTF—ciliary neurotrophic factor; CSA—cross-sectional area; DRG—dorsal root ganglion; FGF—fibroblast growth factor; GAP-43—growth associated protein 43; ICR—Institute of Cancer Research; IFN-γ—interferon-γ; IL—interleukin; MAPK—mitogen-activated protein kinase; MBP—myelin basic protein; MDA—malondialdehyde; MMP2/9—matrix-metalloproteinase-2/9; MNCV—motor nerve conduction velocity; NF-κB—nuclear factor kappa B; NGF—nerve growth factor; NPs—natural products; PAs—plasminogen activators; PCNA—proliferating cell nuclear antigen; PDGF—platelet-derived growth factor; PFI—peroneal function index; PI3K—phosphoinositide 3-kinase; PMP22—peripheral myelin protein 22; Prom1—prominin 1; SD—Sprague-Dawley; SFI—sciatic function index; Sox18—sex-determining region Y-box transcription factor 18; TFI—tibial function index; TGF-β—transforming growth factor-β; TNF-α—tumor necrosis factor-α; tPA—tissue plasminogen activator; Trk—tropomyosin receptor kinase; TRPC1—transient receptor potential cation channel subfamily C member 1; TSI—toe spread index; uPA—urokinase plasminogen activator; Vegf—vascular endothelial growth factor; WRL—withdrawal reflex latency.
2.4.4. Involvement of CAMs in Combinatorial Approaches for the Treatment of PNI
There is increasing evidence that the successful repair and regeneration of nerves will require not just a single treatment strategy, but a multifaceted strategy involving different disciplines. Studies adopting combinatorial approaches have yielded interesting findings. For example, Lycium barbarum polysaccharide incorporated into core-shell structured nanofibrous scaffolds by coaxial electrospinning showed proliferative effects in PC12, SCs, and DRG neurons [87]. In two separate studies, puerarin, the active component extracted from Pueraria lobata roots, as well as rat serum metabolites of P. lobata enhanced the neuroregenerative effects of silicone rubber nerve chambers. Increase in myelinated axons and structurally mature regenerated axons were observed, while muscle reinnervation led to functional recovery, as indicated by an increase in action potential and nerve conduction [89][90]. Similar results were obtained with Buyang Huanwu decoction being administered as a co-treatment alongside silicone rubber nerve chambers, which led to more prominent axonal regeneration [98]. In an SNI model, a magnetic nanocomposite scaffold produced from using magnetic nanoparticles and biodegradable chitosan-glycerophosphate polymer enhanced SC viability, nerve regeneration, and functional recovery when paired with an applied magnetic field [110]. The use of nerve guiding conduits gained popularity over the years. They have been used to isolate regenerating axons from fibrotic tissues, to protect them from mechanical forces, and to guide new-forming tissue as well as condensing growth factors secreted by SCs [111]. The concept was initiated with a simple hollow design but has since advanced to innovative ways of redesigning nerve conduits to further extend their original capabilities 11. The attractive characteristics of modern nerve conduits offer tremendous potentials. These nerve conduits are occasionally paired with other strategies for improving nerve outcomes. For instance, Chang et al. [112] developed a natural biodegradable multi-channeled scaffold with aligned electrospun nanofibers and a neurotrophic gradient, which resulted in superior nerve recovery and less muscle atrophy compared with nerve autografts. Hussin et al. [74] used Centella asiatica (L.) to neurodifferentiate mesenchymal stem cells. This was subsequently developed with decellularized artery as a nerve conduit, which demonstrated functional restoration in an SNI model similar to that of reversed autograft.
3. Conclusions
Peripheral nerve injury remains a challenge, while future prospects are leaning towards multi-combinatorial approaches. Natural products are highly appreciated for their therapeutic value, and there is a growing body of evidence in their potential for peripheral nerve regeneration. The present findings showed that various NPs promote the proliferation and migration of SCs, most commonly through the activation of MAPK and FGF-2 signaling pathways, respectively. Promotion of peripheral nerve regeneration was also observed in rodent models, partly through the modulation of neurotrophic factors, pro-inflammatory cytokines, and anti-apoptotic signaling. Hence, NPs could play key roles in nerve repair and regeneration in the near future, especially when paired with other innovative approaches such as modern nerve conduits.