1000/1000
Hot
Most Recent

Advances in peptide development have made peptide-assisted gene delivery more efficient in vitro and, in some instances, in small animal models. For example, cell and tissue selectivity could be greatly enhanced in the newest generation of CPPs. Other advances which allow for improved performance with regard to targeting and delivery of nucleic acids include adapting peptide sequences to facilitate escape or release from intracellular vesicles or respond to environmental stimuli for a controlled release of cargo, and the development of composite, multivalent peptide-based, or peptide-coupled structures.
Introducing exogenous nucleic acids into human target cells has been receiving a great deal of attention for the treatment of several human diseases, in particular cancer and other genetic disorders. Despite a broad range of possible therapeutic approaches, the clinical success of gene therapy has yet to meet the expectations. The lack of efficacy and issues with clinical safety, in particular with viral vectors, which make up about 70% of vectors used in gene therapy, are the main reasons gene delivery systems fail in clinical trials.[1][2] This has led to the emergence of non-viral vector systems, such as liposomes and polymer supramolecular assemblies with better biological safety. However, their efficacy is predominantly hampered by insufficient localization of the therapeutic agents at the site of interest, both at the extracellular and intracellular level.[3] Owing to their remarkable potency, selectivity and low toxicity, peptides offer ideal alternatives to overcome these hurdles.[4]
The major shortcomings of most commonly used non-viral nucleic acid delivery systems, such as lipoplexes and polyplexes include nonspecific distribution, inefficient cytoplasmic delivery, and organelle targeting. In contrast, peptide-based nanocarriers, e.g., peptide nanoparticles, also called peptiplexes, or peptidic multicompartment micelles, and nano-assemblies equipped with peptides hold great promise as delivery platforms, since they can be tweaked to facilitate penetration of cell membranes and to localize to distinct subcellular compartments. In addition, peptides are easy to synthesize with a desired bioactivity, and, by multivalent presence, endow the nanocarrier with high avidity for the target.[5][6] Owing to the highly specific targeting capacity of corresponding peptides, therapeutic nanocarriers are able to pass through the cell membrane and reach the specific tissue and cells which results in enhanced intracellular distribution and extended therapeutic window.[7] Furthermore, smart delivery systems are promising options to provide solutions related to uncontrolled release of payloads: besides a biocompatible nanocarrier and suitable targeting moieties, these platforms include stimulus-responsive elements which endow them with triggered cargo release.[8]
Membrane active peptides interact with cellular membranes by traversing them, disrupting them or by residing at the membrane interface and fusing with them.[9] They are known to overcome site-specific delivery barriers and facilitate intracellular delivery of various bioactive cargos with low cytotoxicity.[9][10] Although there is a wide variety of membrane-active peptides, here we mainly discuss peptides for targeting nucleic acid delivery systems to specific cells and tissues, and peptides that assist in the delivery of nanocarriers across membrane barriers, such as cell penetrating peptides (CPPs), peptides facilitating endosomal escape and those that target nanocarriers to subcellular organelles.
In view of the fact that the nuclear membrane is the main barrier restricting transgene expression of most non-viral carriers, gene therapy is the obvious field for the application of nuclear targeting peptides.[11] The significance of incorporating NLS peptides into non-viral delivery systems that can adequately favor the genetic materials release into the nucleus manifests itself by expanding case studies.[12][13][14] Hereby, positively charged NLS peptides either are attached to the negatively charged DNA via electrostatic interactions or are covalently coupled to the phosphate backbone of the DNA or to the condensing agent of the non-viral vector.[11]
Peptides have great potential as self-assembly building blocks on account of primary and secondary structure variability. Depending on the design, they form various supramolecular assemblies, such as vesicles,[15] micelles,[16] nanotubes ,[17] nanofibers,[18] or nanoribbons.[19] Choosing corresponding peptide building blocks allows for tuning size and shape of the nano-assembly to obtain improved nanocarrier properties including cargo loading and delivery.[20][21] Moreover, the weak interactions involved in peptide self-assembly are sensitive to environmental conditions, enabling nano-assemblies to exhibit specific functionalities in response to different external stimuli, such as temperature, pH, redox state, enzymes, or even light.
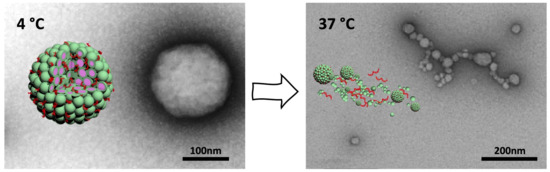
Light has received much attention as an external stimulus, as it provides spatiotemporal control that can be triggered remotely. By crosslinking peptides with specific light-absorbing molecules it is possible to obtain photo-responsive conjugates that allow for light-stimulated assembly of nanostructures or light-induced release of cargo molecules. Such light-sensitive conjugates of peptides and photosensitizers can serve as light-controllable phototherapeutic agents.[25]
In view of gene therapy, endowing nanocarries with targeting features and stimuli-responsiveness that provides site-specific, triggerable control over cargo release could optimize delivery efficacy, and, at the same time, minimize adverse effects. Since the introduction of peptides as potential delivery system for a variety of therapeutic cargos, extensive research has focused on their application in gene therapy. To be suitably tailored for gene therapy, peptide-based nanocarriers must comply with issues of targeting, cellular uptake, and intracellular trafficking, all of which involve biological membranes and how they can be overcome. A combinatorial approach, e.g., designer peptides composed of cationic cell-penetrating and hydrophobic endosomal escape domains in combination with a gene carrier peptide composed of targeting and cationic DNA-binding domains affording triggered, site-specific (cytosol, nucleus, mitochondria) release of nucleic acids, may offer some improvement of efficacy. Other properties, including, but not limited to, low cytotoxicity, target specificity, biodegradability, and cost and time efficiency of synthesis greatly contribute to the potential of peptides in nanomedicine. Nevertheless, to broadly realize bench to bedside translation of peptide-related gene delivery systems, innovative technologies need to be pursued to achieve peptide-based nanocarriers that more specifically and efficiently deliver nucleic acids or nucleic acid modifying systems to the desired sites. In many cases, such nanocarriers would further benefit from either sustained or triggered delivery options.