In recent years, enzymes have risen as promising therapeutic tools for different pathologies, from metabolic deficiencies, such as fibrosis conditions, ocular pathologies or joint problems, to cancer or cardiovascular diseases. Treatments based on the catalytic activity of enzymes are able to convert a wide range of target molecules to restore the correct physiological metabolism. These treatments present several advantages compared to established therapeutic approaches thanks to their affinity and specificity properties. However, enzymes present some challenges, such as short in vivo half-life, lack of targeted action and, in particular, patient immune system reaction against the enzyme. For this reason, it is important to monitor serum immune response during treatment. This can be achieved by conventional techniques (ELISA) but also by new promising tools such as microarrays. These assays have gained popularity due to their high-throughput analysis capacity, their simplicity, and their potential to monitor the immune response of patients during enzyme therapies. In this growing field, research is still ongoing to solve current health problems such as COVID-19. Currently, promising therapeutic alternatives using the angiotensin-converting enzyme 2 (ACE2) are being studied to treat COVID-19.
1. Introduction
Enzymes are chemical catalysts of biological systems. They allow organisms to self-replicate and catalyze, in a selective and efficient manner, essential biochemical reactions. Enzymes are proteins, except for ribozymes, which are a small group of RNA molecules with a catalytic activity
[1]. These proteins have a high specificity that allows them to discriminate between substrates with similar structures
[2]. Furthermore, they possess an extraordinary catalytic power that accelerates the targeted chemical reactions. The process of catalyzing biochemical reactions takes place in aqueous solutions under very mild conditions of temperature and pH
[3].
Enzymes are essential in biochemical processes. They catalyze hundreds of stepwise metabolism reactions, preserving and transforming chemical energy and generating biological macromolecules from precursors. Their catalytic activity depends on the integrity of their native protein conformation. In this regard, the activity of one or more enzymes is impaired in many diseases due to mutations
[4]. Because of the necessity of the correct performance of the enzymes, many drugs have been developed with the aim to target dysfunctional enzymes
[5].
An alternative approach is to use enzymes directly as therapeutic drugs. They were used firstly at the end of the 19th century, when enzymes such as pepsin were used to treat dyspepsia
[6].
In 1987, the first recombinant enzyme drug for acute ischemic stroke, plasminogen activator Alteplase, was approved by the Food and Drug Administration (FDA, Montgomery, MD, USA)
[7]. This drug was prescribed for the treatment of acute ischemic stroke thanks to its capacity to dissolve clots and restore tissue perfusion
[8]. To support the growing demand for these enzymatic treatments, major efforts are being invested in their industrial production, using recombinant expression of these molecules in plants, mammalian systems and microbial systems (fungi, yeast or bacteria)
[9]. However, some enzyme drugs are taken directly from nature, for instance, snake venom
[10][11].
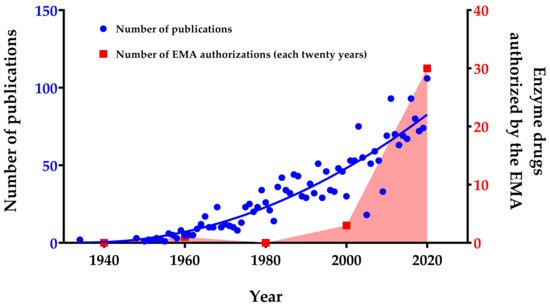
The industrial market for enzyme-based drugs is expected to increase at a compound annual growth rate of 6.8% within the period 2019–2024
[12]. In 2024, markets that involve proteases or carbohydrase are estimated to reach 2 and 2.5 billion USD, respectively
[13]. These market indicators are reflected in an increase in the number of enzyme drugs authorized in recent years (
Figure 1). Together with this economic growth, an increase in the number of publications concerning enzyme therapy has been observed, highlighting the growing interest and potential of this field (
Figure 1). The observed increase in research publications and patents to date highlights the efforts invested in this field mainly because of the promising therapeutic potential of enzymes. Presently, enzymes are not only being used and investigated for the treatment of metabolic deficiencies but also for many different pathologies such as cancer and cardiovascular diseases
[14][15][16][17].
Figure 1. Number of publications and enzyme drugs authorized per year (from 1934 to 2020). Publication searches were performed by entering the subject “enzyme therapy, drug and treatment” in PubMed database and choosing the field “Title/Abstract” to filter the search. The enzyme drug searches were performed in the European Medicines Agency (EMA, Amsterdam, The Netherlands) database, and the number of authorized enzymes per twenty-years’ time intervals was plotted. Red area only highlights the growing trend in the number of enzymes authorized by the EMA.
The potential of enzyme-based drugs can be improved in regard to specific factors. First, the in vivo half-life of the molecules should be improved; second, the targeted action is not always accurate; and third, valid methods are necessary to control the patient’s immune system response during treatments based on enzymes
[18]. In this context, novel approaches to monitor the immune response, such as microarrays, are of ongoing interest for personalized medicine. Moreover, newer approaches based on enzymes are being studied to treat infections such as SARS-CoV-2 and its associated pathology, COVID-19, highlighting the potential benefit of enzyme therapy.
2. Enzyme Therapies for Different Pathologies
Since their first uses as drugs, enzymes have been widely applied to treat enzymatic deficiencies and several health issues.
Therapies based on enzymes can be systemic or non-systemic, and they have multiple administration routes: oral
[19], topic
[20], respiratory
[21] or intravenous
[22]. We classified the main pathologies treated with enzymes according to the type of disease. A summary of the categorization is included in the table at the end of this section (
Table 1), which will also be referenced in the subsections for each type of disease.
Table 1. Summary of the main pathologies and conditions treated with enzymes.
Disease/
Condition |
Cause/Pathology |
Therapeutic Enzymes * |
Ref. |
| Lysosomal storage diseases |
| Gaucher’s disease |
Deficiency of glucocerebrosidase |
Glucocerebrosidase [Cerezyme, Vprip, Taliglucerase alpha] |
[23], (a,b,c) |
| Hunter’s syndrome |
Deficiency of iduronate-2-sulfatase |
Iduronate-2-sulfatase [Elaprase] |
[24], (d) |
| Fabry’s disease |
Deficiency of α-galactosidase A |
α, β-galactosidase A [Replagal, Fabrazyme] |
[25], (e,f) |
| Hurler’s syndrome |
Deficiency of α-L-iduronidase |
α-L-iduronidase [Aldurazyme] |
[26], (g) |
| Morquio syndrome type A |
Deficiency of N-acetylgalactosamine-6-sulfate sulfatase |
N-acetylgalactosamine-6-sulfate sulfatase
[Vimizim] |
[27], (h) |
Maroteaux-Lamy
syndrome |
Deficiency of arylsulfatase B |
N-acetylgalactosamine-4-sulfatase [Naglazyme] |
[28], (i) |
| Sly syndrome |
Deficiency of β-glucuronidase |
β-glucuronidase [Mepsevii] |
[29], (j) |
| α-Mannosidosis |
Deficiency of α-D-mannosidase |
Velmanase α [Lamzede] |
[30], (k) |
| Batten disease |
Deficiency of tripeptidyl
peptidase 1 |
Cerliponase α [Brineura] |
[31], (l) |
| Pompe’s disease |
Deficiency of acid α-glucosidase |
α-glucosidase [Myozyme] |
[32], (m) |
| Metabolic deficiencies |
| Exocrine pancreatic insufficiency (EPI) |
Insufficient secretion of
pancreatic enzymes |
Pancreatic enzymes [Enzepi] |
[33][34][35], (n) |
| Phenylketonuria (PKU) |
Deficiency of phenylalanine
hydroxylase (PAH) |
PAH and phenylalanine ammonia-lyase PAH [Palynziq] |
[36], (o) |
| Severe combined immunodeficiency (SCID) |
Deficiency of adenosine
deaminase (ADA) |
Polyethylene glycol-conjugated ADA |
[37][38] |
| Wolman disease |
Deficiency of lysosomal
acid lipase |
Lysosomal acid lipase [Kanuma] |
[39], (p) |
| Acute intermittent porphyria (AIP) |
Deficiency of hydroxymethylbilane synthase |
Hydroxymethylbilane synthase and porphobilinogen deaminase |
[40] |
| Congenital sucrase-isomaltase deficiency (CSID) |
Deficiency of sucrase and
isomaltase |
Sacrosidase |
[41] |
| Hypophosphatasia |
Deficiency of tissue-nonspecific isoenzyme of alkaline phosphatase (TNSALP) |
TNSALP [Strensiq] |
[42], (q) |
| Protein C deficiency |
Deficiency of Protein C |
Protein C [Ceprotin] |
[43], (r) |
| Lactose intolerance |
Reduction or loss of the activity of lactase-phlorizin hydrolase |
Lactase |
[44] |
| Fibrosis conditions |
| Chronic total occlusions |
Fibrous plaques obstructing
coronary arteries |
Collagenase Clostridium histolyticum (CCH) |
[45] |
| Dupuytren’s disease |
Thickening of the fascia tissue in the hands |
Collagenase Clostridium histolyticum (CCH) [Xiapex] |
[22][46], (s) |
| Peyronie’s disease |
Fibrous plaques formation in the penis |
Collagenase Clostridium histolyticum (CCH) |
[20] |
| Uterine fibroid |
Fibroid tissue growth around the uterus |
Collagenase Clostridium histolyticum (CCH) |
[47] |
| Keloid disease |
Overgrowth of granulation scar tissue |
Collagenases and matrix metallopeptidases |
[48][49] |
| Lung cystic fibrosis |
Viscose secretions in the lungs |
Deoxyribonuclease I [Pulmozyme] |
[21], (t) |
| Glaucoma |
Fibrous formations at the
trabecular meshwork of the eye |
Collagenases |
[50] |
| Ocular affections |
Different ocular
diseases treated with vitrectomy |
Malfunction of the vitreous
humor of the eye solved by its
enzymatic removal |
Chondroitinase, hyaluronidase, nattokinase and ocriplasmin [Jetrea] |
[51], (u) |
| Joint problems |
Intervertebral disc
herniation |
Disc material penetrating
the spinal dura |
Chondroitin sulfate ABC endolyase |
[52] |
| Arthritis |
Osteophytes formation and
inflammation |
Proteolytic enzymes |
[53][54] |
| Cancer |
Different types of
cancer |
Increased amino acid metabolism in the tumor microenvironment |
PEGylated arginine deaminase and kynureninase [Voraxaze, PEG hyaluronidase PH20] |
[14][55], (v,w,x) |
| Leukemia |
Increased amino acid metabolism in the tumor microenvironment |
L-asparaginase [Spectrila, Kidrolase, Erwinase, Oncaspar] |
[16][55], (y,z) |
| Chemotherapy-induced hyperuricemia |
Increase in uric acid due to tumor lysis syndrome |
Urate oxidase and rasburicase [Fasturtec] |
[56], (aa) |
| Cardiovascular diseases |
| Cardiovascular disease |
Formation of fibrin clots degraded by plasmin |
Nattokinase and urokinase [Streptase, Syner-Kinase, Kinclytic, Rapilsyn,
Actilyse, Metalyse] |
[17], (ab,ac,ad,ae,af) |
| Extracellular matrix disorders |
| Burns |
Denatured collagen in necrotic tissue |
Collagenase Clostridium histolyticum (CCH) [Nexobrid] |
[57][58], (ag) |
| Cellulite |
Accumulation of subdermal
collagen in the dermal septa |
Collagenases |
[59] |
| Reactive oxygen species damage |
Organ injury in
hemorrhagic shock |
Reactive oxygen species (ROS) tissue damage |
Superoxide dismutase |
[60] |
| Parkinson’s |
Reactive oxygen species (ROS) tissue damage |
Nanozyme (PtCu nanoalloys) |
[61] |
| Other applications |
| Celiac disease |
Gluten intolerance |
Gluten-degrading enzymes |
[62] |
| Microbial infections |
Microbial biofilm formation
during infection |
Matrix-degrading enzymes (polysaccharide-degrading enzymes, nucleases and proteases) |
[63] |
| Inflammation |
Inflammation of overexpressed pathways disrupting
physiological homeostasis |
Proteolytic enzymes
(trypsin or serratiopeptidase) |
[64][65] |
| Cocaine overdose |
Cocaine toxicity |
Human butyrylcholinesterase (BChE) or
Bacterial cocaine esterase (CocE) |
[66] |
* Tradenames of the enzymes are given in brackets. Lowercase letters reference to enzyme drugs authorized by the EMA.
2.1. Metabolic Deficiencies
Pathologies caused by the absence or deficiency of an enzyme are the main targets for enzyme replacement therapy (ERT). These medical treatments are employed to try to restore the lost or altered enzymatic activity. Usually, the enzyme is administrated through an intravenous solution. The main metabolic deficiencies treated with ERT are the lysosomal storage diseases (LSD).
2.1.1. Lysosomal Storage Diseases (LSD)
LSD are a heterogeneous group of rare inherited metabolic disorders that are the result of lysosomal dysfunctions. They originate from a deposit of noncatalyzed glycosaminoglycans, which is caused by a deficiency in lysosomal enzymes or alterations in molecular transport. Gaucher’s disease, Hunter’s syndrome, Fabry’s disease, Hurler’s syndrome, Morquio syndrome type A, Maroteaux-Lamy syndrome, Sly syndrome, α-mannosidosis, Batten disease and Pompe’s disease are examples of disorders included in the LSD group. At the moment, some biomarker discovery projects are underway to improve LSD diagnosis
[67][68]. Due to the features of the above-mentioned pathologies, ERT appears to be a promising therapeutic alternative. A summary of LSD treated with enzyme drugs is showed in
Table 1.
Gaucher’s disease is caused by the loss of the glucocerebrosidase enzyme, which leads to the accumulation of lipids, such as glucocerebroside, especially in the bone marrow, spleen and liver. As a consequence, swollen liver and/or spleen, anemia, thrombocytopenia and skeletal abnormalities can be present in affected patients. In this context, ERT is able to balance the low levels of glucocerebrosidase with the administration of a recombinant version of the enzyme through intravenous injections
[23].
Hunter’s syndrome, also known as Mucopolysaccharidosis type II, is a rare and inherited pathology triggered by the deficiency of iduronate 2-sulfatase (I2S), an enzyme catalyzing the degradation of the glycosaminoglycans dermatan- and heparan-sulfate. In the absence of these enzymes, molecules accumulate in organs and tissues, leading to an imbalance in normal homeostasis that can influence physical and mental development. In these cases, recombinant I2S is administrated intravenously as an ERT, leading to improvement of the clinical parameters
[24].
Fabry’s disease is a rare and inherited condition triggered by a deficiency of the lysosomal enzyme α-galactosidase A (AGAL). Thus, a progressive deposition of an incomplete metabolized lipid substrate (Gb3) is observed in multiple cell types, causing alterations in vascular reactivity and a propensity for thrombo-embolic disease
[69]. These abnormalities are believed to play a role in increased risk for particular problems, with renal and cardiac failure being the main causes of morbidity
[69]. An intravenous infusion of a recombinant form of AGAL as ERT can improve the course of the disease
[25].
Hurler’s syndrome, Morquio syndrome type A, Maroteaux-Lamy syndrome, Sly syndrome, α-mannosidosis, Batten disease and Pompe’s disease are other examples of LSD, characterized by α-L-iduronidase, N-acetylgalactosamine-6-sulfate sulfatase, arylsulfatase B, β-glucuronidase, α-D-mannosidase, tripeptidyl peptidase 1 and acid α-glucosidase deficits, respectively. To treat these pathologies, ERT represents the best therapeutic approach
[26][27][28][29][30][31][32].
2.1.2. Further Metabolic Deficiencies
In addition to LSD, there are several other metabolic deficiencies that need to be considered (Table 1).
Exocrine pancreatic insufficiency (EPI) is characterized by an impaired secretion of pancreatic enzymes and bicarbonate. EPI can be caused by upper gastrointestinal and pancreatic surgery as well as by different pancreatic diseases, such as cystic fibrosis (CF). The consequent maldigestion and malabsorption of nutrients leads to several nutritional deficiencies. To improve patients’ quality of life, pancreatic ERT represents a valid approach
[33][34]. However, nutrient malabsorption has also been observed in acquired immunodeficiency syndrome (AIDS), whose related experimental studies have shown promising results for the use of pancreatic ERT in the improvement of this condition
[35].
Phenylketonuria (PKU) is an inborn disease caused by mutations in the phenylalanine hydroxylase (PHA) gene. These alterations lead to an enzyme deficiency that causes hyperphenylalaninemia. One of the approaches to control phenylalanine concentration is to use a PHA ERT. For this purpose, unmodified PHA and phenylalanine ammonia-lyase PHA can be administrated
[36].
Severe combined immunodeficiency (SCID) is a group of rare pathologies, in which the genes involved in the development and function of immune cells are mutated. One subtype of SCID is characterized by adenosine deaminase (ADA) enzyme deficiency. The function of this enzyme is necessary for the breakdown of adenosine absorbed from food and for the turnover of nucleic acids in tissues. Its insufficiency leads to the accumulation of toxic purine degradation products, which mostly affect lymphocytes, causing immunodeficiency. ERT based on polyethylene glycol-conjugated adenosine deaminase (PEG-ADA) shows an improved life quality
[37]. PEG modifications reduce the plasma clearance of the enzyme, as they decrease cellular uptake, proteolysis and immunogenicity compared to the unmodified enzyme. As a consequence, circulating levels and the in vivo half-life of the therapeutic enzyme are improved
[38].
Many other metabolic diseases in which ERT can play a crucial role are mentioned below. Wolman disease, which is characterized by the absence of the lysosomal acid lipase (LAL) enzyme, could be treated by administrating LAL as an ERT
[39]. In acute intermittent porphyria (AIP), the deficiency of the enzyme hydroxymethylbilane synthase (HMBS), also known as porphobilinogen deaminase (PBGD), could be addressed by administrating an ERT based on HMBS/PBGD
[40]. Furthermore, congenital sucrase-isomaltase (SI) deficiency (CSID) is the result of a reduction or loss of the SI enzyme, which could be treated with an ERT by administrating Sucraid (sacrosidase)
[41]. In cases with hypophosphatasia, which is a disease characterized by the tissue-nonspecific isoenzyme of alkaline phosphatase (TNSALP) deficiency, TNSALP ERT represents a valid treatment
[42]. Protein C deficiency can also be treated with ERT by administering the protein
[43]. Lastly, ERT can also be used in cases of lactase deficiency by delivering microbial recombinant lactase
[44].
2.2. Fibrosis Conditions
Interest in peptidase enzymes is increasing due to their capacity to degrade protein deposits in different types of tissues. Metalloprotease endopeptidases, which include collagenases and gelatinases (such as matrix metallopeptidase, MMP, 9 or 2), are being studied as treatments for different pathologies. Table 1 presents a synopsis of the different fibrosis conditions treated with enzymes.
Chronic total occlusion (CTO) is a complete or partially complete obstruction that concerns coronary arteries. The blockage is produced by the accumulation of a collagen plaque in a coronary artery, which could compromise blood flow to the heart. One of the current therapies is the local administration by catheter of type IA collagenase, a bacterial collagenase formulation obtained from Clostridium histolyticum (CCH, Collagenase Clostridium histolyticum) which is able to degrade the collagen plaques
[45]. Furthermore, CCH is administrated also in Dupuytren’s disease for the enzymatic removal of the fibrotic fascia (fasciotomy). This pathology is characterized by the thickening of the fascia, which is the fibrous layer of tissue that lies underneath the skin of the palm and fingers. As a result of this abnormality, hands present some deformations
[22][46]. Lastly, CCH is also applied for the enzymatic digestion of fiber plaques and fiber tissue found in Peyronie’s disease
[20] and Uterine fibroids
[47], respectively.
Keloids, lung CF and glaucoma are further examples of fibrosis conditions that can be treated with enzymes. Keloids are fibroproliferative dermal tumors with effusive accumulation of extracellular matrix that can generate after surgery. Collagenases and matrix metallopeptidases have been demonstrated to be safe and efficient in reducing keloids
[48][49]. Moreover, lung CF is a pathology caused by the formation of thickened mucus in the lungs. A recombinant form of deoxyribonuclease I (Dornase α) can be administrated to dissolve the secretions
[70]. Glaucoma represents a group of eye conditions that damage the optic nerve, leading to diverse vision problems, and are potentially able to cause blindness. In many cases, fibrosis is known to occur as a consequence of extracellular matrix accumulation in the trabecular meshwork at the anterior part of the eye and in the lamina cribrosa at the optic nerve head. A novel method to reduce fibrosis through administration of purified collagenase into a patient eyes has been patented
[50].
2.3. Ocular Affections
Retinal detachment, macular pucker, diabetic retinopathy, macular holes, vitreous hemorrhage and vitreous floaters are ocular pathologies that can be treated with a vitrectomy, which is a surgery to remove some or all the vitreous humor of the eye. However, the use of enzymes, such as chondroitinase, hyaluronidase, nattokinase or ocriplasmin, allows the non-invasive removal of the vitreous humor simply by digestion
[51] (
Table 1).
2.4. Joint Problems
Different conditions related to chronic and pathological joint problems, associated with pain and inflammation, are being treated with enzymes (Table 1).
Intradural disc herniation (IDH) occurs when disc material penetrates the spinal dura and lies in an extramedullary location. IDH can be treated with chemonucleolysis by injecting an enzyme into the vertebral disc, aiming to dissolve its inner part. Sulfate ABC endolyase, an enzyme that catalyzes the depolymerization of chondroitin sulfate, is used for this purpose
[52].
Arthritis, especially osteoarthritis and rheumatoid arthritis, is a pathology that causes pain and inflammation in a joint. Anti-inflammatory drugs, combined with proteolytic enzyme supplements, show diminished pain and improved quality of life
[53][54].