1000/1000
Hot
Most Recent

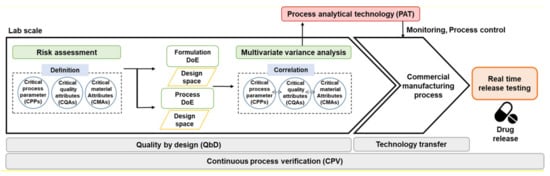
Various frameworks and methods, such as quality by design (QbD), real time release test (RTRT), and continuous process verification (CPV), have been introduced to improve drug product quality in the pharmaceutical industry. The methods recognize that an appropriate combination of process controls and predefined material attributes and intermediate quality attributes (IQAs) during processing may provide greater assurance of product quality than end-product testing. The efficient analysis method to monitor the relationship between process and quality should be used. Process analytical technology (PAT) was introduced to analyze IQAs during the process of establishing regulatory specifications and facilitating continuous manufacturing improvement. Although PAT was introduced in the pharmaceutical industry in the early 21st century, new PAT tools have been introduced during the last 20 years.
| Process | Critical Process Parameter |
Intermediate Quality Attributes |
Justification | Ref | |
|---|---|---|---|---|---|
| Blending | Blending time |
|
If the blending time is long, separation may occur depending on the particle characteristics, which may affect the content and content uniformity of the mixture. | [25][26] | |
| Blending speed |
|
When blending above the optimum blending speed, the particles adhere to the wall of the blender by centrifugal force, which may affect the uniformity of the content of the mixture. | [25][26][27][28][29] | ||
| Order of input |
|
The order of input of additives has little effect on content and content uniformity because of the blending process in the blender. However, the effect of the input of the lubricant may affect the content and content uniformity. | [26] | ||
| Environment |
|
If temperature and humidity are not controlled, it may affect the moisture content of the mixture, and the content and content uniformity may be affected depending on the moisture and thermal stability of the drug. | [26] | ||
| Filling level |
|
Since the charging rate affects the movement of the particles, it can cause blending non-uniformity. This can affect the content and content uniformity of the mixture. | [25][26][27] | ||
| Granulation | High-shear granulation | Binder solvent amount |
|
When the amount of liquid increases, the powder is completely wetted, which impedes the particle flow in the granulator, which can affect the particle-size distribution of the granules by increasing the residence time and torque value. When the amount of liquid is insufficient, weak granules are formed. | [30][31] |
| Binder solvent concentration |
|
The concentration of the binding liquid has a direct relationship with the binding force and can affect the density and particle-size distribution of the granules. | [32][33][34] | ||
| Binder solvent spray rate |
|
The binder solvent spray rate is directly connected to the size of the granules. If it is too slow, the process time is lengthened, and it is difficult to form granules; if it is too fast, a mass may be formed. Therefore, it can affect the granule-size distribution and density. | [35][36][37][38] | ||
| Filling level |
|
The filling level affects the movement of particles in the granulator ball, so that fine granules may be generated due to an increase in the number of collisions between the granules and an increase in strength. This can affect the content and uniformity of the granules. | [39][40] | ||
| Impeller speed |
|
The speed of the impeller determines the state of the granules. Accordingly, the porosity and density of the granules may be affected, and the particle-size distribution and flowability of the granules may be affected. In addition, as the impeller speed increases, it may affect the granule growth due to coalescence, so it may affect the granule size. | [30][35][41][42][43][44][45] | ||
| Chopper speed |
|
Since the chopper speed plays a role in breaking the mass of granules, it can affect the density of the granules, the particle-size distribution, and the flowability of the granules. | [30][37][41][46] | ||
| Massing time |
|
The massing time is a factor that determines the main physical properties of the granules. Depending on the massing time, the strength of the granules and the density of the granules can be affected, and thus, the flowability and particle-size distribution can also be affected. Excessive massing time can result in granule growth by coalescence, which can affect granule size. Accordingly, it may affect the content uniformity of the granules, which may affect formation of granules. | [31][36][41][47][48][49] | ||
| Mill screen size |
|
The mill screen size can affect the physical properties of the granules, such as the density and flowability of the granules, due to a large correlation with the particle-size distribution of the granules. | [35] | ||
| Nozzle type |
|
The nozzle position affects the spray angle of the binder solvent, which can affect the agglomeration and growth of the granules, but the effect is negligible. In addition, the size of the nozzle hole affects the distribution of the binder solution. However, this has little effect when adjusted with other process variables. | [39][50] | ||
| Fluidized-bed granulation | Binder amount |
|
When the amount of liquid increases, the powder is completely wetted, which impedes the particle flow in the granulator, which can affect the particle-size distribution of the granules by increasing the residence time and torque value. When the amount of liquid is insufficient, weak granules are formed. | [51] | |
| Binder concentration |
|
The concentration of the binding liquid has a direct relationship with the binding force and can affect the density and particle-size distribution of the granules. | [52][53][54][55][56][57] | ||
| Binder spray rate |
|
The binder solvent spray rate is directly connected to the size of the granules. If it is too slow, the process time is lengthened and it is difficult to form granules; if it is too fast, a mass may be formed. Therefore, it can affect the granule-size distribution and density. | [53][54][55][56][57][58][59][60] | ||
| Air volume/temperature/humidity |
|
Higher temperature increases fineness due to rapid drying, and lower temperature causes granules to agglomerate, resulting in harder and larger granules. This can affect the density, flowability and particle-size distribution of the granules. The flow of particles is determined according to the air-supply flow rate, and if it is too high, the degree of blending due to process loss may be lowered, which may affect the density, flowability, and particle-size distribution of the granules. The air-supply humidity determines the size of the granules, which can affect the particle-size distribution of the granules. | [52][53][59][61] | ||
| Nozzle position |
|
The position of the nozzle affects the spray angle of the binder solvent, which can affect the agglomeration and growth of the granules, but the effect is negligible. | [54] | ||
| Nozzle type |
|
The nozzle type affects the way the binder is sprayed into the fluidized-bed of the particles, which can affect the particle-size distribution or density of the granules. | [54][62] | ||
| Drying temperature/time |
|
It can be determined according to the heat and moisture stability of the drug. If the drying time is short or the granules are not sufficiently dried due to the low drying temperature, the moisture content of the granules may be affected. If it is too high, fines may occur due to over-drying, which may affect the flowability and density of the particles. | [59][61] | ||
| Environment |
|
If the temperature and humidity are not managed, it may affect the moisture content of the granules, and the moisture and thermal stability of the drug may affect the content and content uniformity of the granules. | [59][63] | ||
| Twin-screw granulation | Binder viscosity |
|
When the binder solvent viscosity is high, there is a risk of granule mass, which may affect the size and particle-size distribution of the granules. | [64] | |
| Liquid to solid ratio |
|
If the amount of liquid inside the granulator increases, the powder may become excessively moistened and impede the flow of the inside. This increases the residence time and can thus affect the size and particle-size distribution of the granules. | [65][66][67][68][69] | ||
| Feeder rate |
|
The feed rate of the powder affects the residence time, and due to the low feed rate, the inside of the granulator is not completely filled, and the residence time may be lengthened. This can affect granule properties, such as the particle-size distribution, density and flowability of the granules. | [65][66][67][70] | ||
| Screw speed |
|
The screw speed can affect the residence time and, accordingly, the particle-size distribution and density of the granules. | [63][65][66][67][71][72][73][74] | ||
| Screw type |
|
The type of screw is affected by the shape and angle of the screw to be engaged or the kneading pattern of the kneader part. This affects the amount of filling inside the granulator and can directly affect the compression and crushing of agglomerated particles and the distribution of the granules. | [65][66][69][75] | ||
| Filling level |
|
The feeder amount is directly related to the residence time and can affect the particle-size distribution and density of the granules. | [65][71] | ||
| Residence time |
|
The residence time of the powder can affect the size and particle-size distribution of the granules. | [66][72][75] | ||
| Roller compaction | Roller compactor type |
|
Depending on the type of roller compactor, the principle of operation is different, which can affect the properties of the ribbon and the powdery properties of granules (roller width, roller diameter). The larger the diameter of the roller, the larger the compression area, so it may affect the characteristics of the ribbon, but, in general, the diameter of the roller is used as a fixed factor, so the effect on the intermediate product is insignificant. | [76] | |
| Roller pressure |
|
Since the roller pressure determines the bonding force of the powder, it is judged to be directly related to the density of the ribbon. This may affect granule particle-size distribution, flowability and content uniformity after mill screening. | [35][76][77][78][79][80] | ||
| Roller speed |
|
The roller speed is controlled by the screw speed, and it is judged that it has a direct relationship with the density of the ribbon as well as controlling the speed of the process. This affects the powder properties of the granules, which can affect the particle-size distribution and flowability of the granules. | [35][78][80][81][82][83][84] | ||
| Roller gap |
|
The roller gap affects the bonding force of the powder fed into the feeder, and may affect the ribbon density. This affects the powder properties of the granules after mill screening, which may affect the particle-size distribution and flowability of the granules. As the width of the roller changes, it is directly related to the maximum pressure of the roller, which can affect the density of the ribbon and thus the density and particle-size distribution of the granules. | [35][76][78][79][81][83] | ||
| Feeder rate |
|
Input speed is directly related to roller pressure or roller spacing, which can affect the ribbon density, particle-size distribution and flowability of the granules. | [79][82] | ||
| Feed screw speed |
|
Feed screw speed is a variable that is affected by roller pressure and roller spacing, and the effect is negligible. | [80] | ||
| Residence time |
|
The residence time of the powder can affect the size and particle-size distribution of the granules. | [85][86][87] | ||
| Mill screen size |
|
The size of the granulator can affect the physical properties of the granules, such as the density and flowability of the granules, due to a large correlation with the particle-size distribution of the granules. | [76][78][88] | ||
| Mill speed |
|
The speed of the granulator can affect the powdery properties of the granules, but the effect is insignificant. | |||
| Drying process |
Drying time |
|
If the drying time is short, and the result is not fully dried, the moisture content may be affected. If the drying takes too long, fine powder may be generated due to over-drying, which may affect the flowability and distribution of the particles. | [89][90][91] | |
| Drying temperature | If the drying temperature is low, and the result is not fully dried, the moisture content may be affected. If the drying temperature is too high, fine powder may be generated due to over-drying, which may affect the flowability and particle distribution of the particles. | ||||
| Inlet air temperature |
|
The thermal charge of the inlet drying gas reflects its capacity to dry the humid atomized droplets, and, therefore, higher inlet temperatures enable higher solvent evaporation rates. | [92] | ||
| Air flow rate |
|
The flow of particles is determined according to the air-supply flow-rate, and the air-supply flow-rate determines the size of the granules. This can affect the density and particle-size distribution. In addition, an increase in the air flow rate causes a higher evaporation rate. | [89][93] | ||
| Coating process | Rotation speed |
|
As the speed increases, the tablets apparently tumble through the spray zone rather than sliding flat, so the end exposure is more frequent, and the coating becomes more uniform. | [94][95][96] | |
| Nozzle diameter |
|
The size of the sprayed droplet varies depending on the nozzle diameter. Therefore, since the amount of the coating liquid to be sprayed varies, this affects the moisture content and residual solvent. | [97][98] | ||
| Inlet air temperature |
|
If the inlet air temperature is high, the tablets are excessively dried, and the surface becomes rough. If the inlet air temperature is low, the tablets stick together, and the moisture content of the tablets increases. Moisture content and coating uniformity are highly dependent on the incoming air temperature. | [99] | ||
| Air flow rate |
|
The air flow rate prevents the sprayed coating solution from reaching the tablet. The faster the air flow, the lower the velocity of the sprayed droplet and the smaller the droplet size. Therefore, it affects the coating efficiency. | [100] | ||
| Air volume |
|
An improper air layer due to worn or uneven drying may cause agglomeration between particles. An increase in air volume causes a decrease in spray density because the spray area increases as the droplet size decreases at the center of the spray. | [101] | ||
| Coating solution composition |
|
In the case of functional coatings, the coating solution must contain an appropriate composition to deliver the desired effect of the drug, which affects the efficacy of the finished product. In addition, if the ratio of solids constituting the coating solution is high, efficient spraying becomes difficult, thus affecting the coating efficiency. | [102][103] | ||
| Spray rate |
|
Too high a spray rate cause inadequate drying, twining, and sticking. Therefore, spray rate will have a significant impact on surface roughness and weight gain, thus affecting the coating uniformity. | [96][99] | ||
| Atomizing air pressure |
|
Too high a spray pressure can lead to spray drying, and too low can cause agglomeration, which can have a significant impact on coating uniformity. | [104][105][106] | ||
| Curing temperature/ time |
|
The incorrect setting of the curing temperature and curing time will result in incomplete film formation. Thus, full film formation occurs when exposed to a certain curing temperature. The proper setting of curing time is necessary to achieve complete film adhesion. | [107][108][109] | ||
| Tableting process | Feeder speed |
|
Low feeder speeds can lead to improper die filling, which can lead to weight changes and changes in hardness and thickness. Fast feeder speeds can overfill the die cavity and lead to weight variations and hardness and thickness variations. | [110] | |
| Rotary speed |
|
Rotary speed affects compressibility and even affects weight variation, which can affect drug content. A high rotary speed causes a much wider distribution of lubrication extent compared to the results from a low rotary speed. This may induce greater variability in hardness between tablets. | [111] | ||
| Precompression force |
|
Increasing compression force causes difficult particle rearrangement, deformation and fragmentation. Compression force affect tablet porosity, hardness, and density. In addition, depending on the tablet porosity, the degree to which moisture permeates into the tablet varies. | [112][113][114][115][116] | ||
| Main compression force |
|||||
| Dwell time |
|
If the pressure holding time is too long, it deviates from the feeder speed, and inconsistent granules are filled into the die, which may cause weight fluctuations and affect the bonding force of the granules. | [110][111][117][118][119] | ||
| Ejection force |
|
The optimal compression force must be determined to obtain the desired tablet hardness | [120] | ||