1000/1000
Hot
Most Recent

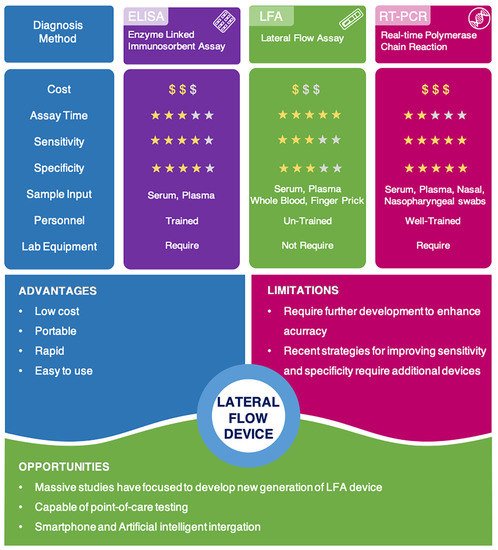
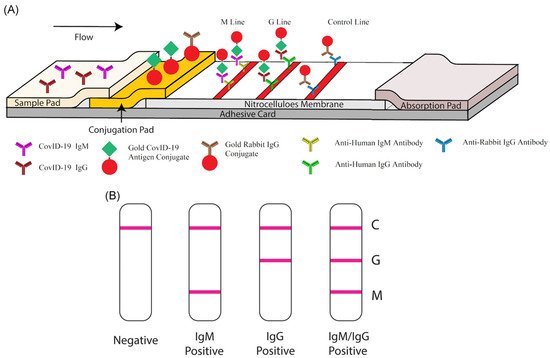
Lateral flow technology (also known as lateral flow assay) plays a critical role in POC testing, as the technique is rapid, cost-effective, and can be operated by untrained personnel. Lateral flow technologies can be classified as follows: lateral flow immunoassay (LFIA), nucleic acid lateral flow assay (NLFA), and nucleic acid lateral flow immunoassay (NALFIA). LFIA is able to detect antibodies/antigens, while NLFA uses a DNA or RNA probe to detect nucleic acid. Moreover, NALFIA uses both antibodies/antigens and nucleic acid as biomarkers for the detection of antigens/antibodies or amplicons.
Accurate and effective diagnosis at COVID-19′s early stages is critical for reducing the risk of transmission, as it allows for quick isolation, contact tracing, and earlier treatment. An ideal diagnostic technique would be cost-effective, portable, rapid, and robust with high sensitivity and specificity [1][2]. This would allow for point-of-care (POC) testing and patient self-administration, resulting in rapid and adequate results and better epidemiological surveillance.
Currently available diagnostic techniques for COVID-19 are based on the detection of the viral gene, antigen, or human antibodies (serological test) and human metabolites [3][4][5][6][7][8][9][10]. Among these techniques, the detection of viral RNA sequences by reverse transcription polymerase chain reaction (RT-PCR), reverse transcription loop-mediated isothermal amplification (RT-LAMP), and reverse transcription quantitative polymerase chain reaction (RT-qPCR) have been the most reliable methods. RT-qPCR uses signal amplification to achieve a high degree of accuracy [11][12][13]. RT-LAMP is a newly established technique in which amplification occurs at a single temperature [14][15][16]. RT-qPCR is able to directly detect SARS-CoV-2 by monitoring the amplification of a targeted DNA molecule during the PCR [7]. Moreover, some novel technologies for detecting viral gene, such as next-generation sequencing (NGS) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), draw great attention due to their better accuracy and higher throughput [17][18]. However, these methods are expensive, time-consuming, and limited to well-trained professional operators. Therefore, they are often not amenable to extensive population-based or POC testing [19][20].
Virus antigens or host antibodies can also be detected serologically. The enzyme-linked immunosorbent assay (ELISA) is a rapid and inexpensive technique for detecting specific antibodies in blood samples. In a recent study, an ELISA test was used to detect human SARS-CoV-2 seroconverters [21]. This test enabled the detection of distinct antibody types as early as three days after the onset of symptoms. However, similar to RT-PCR techniques, the ELISA method also needs to be performed by well-trained personnel. It also relies on specialized equipment, making it difficult to use at POC testing.
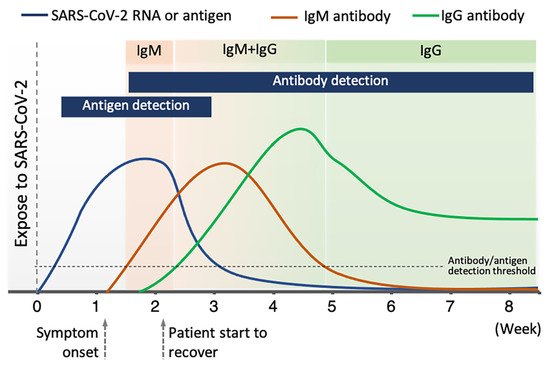
Among available POC testing techniques, the lateral flow immunoassay (LFIA) has been extensively researched and used for COVID-19 diagnosis, owing to its low cost, speed, and accessibility [7][8][19]. To diagnose COVID-19, lateral flow tests combine SARS-CoV-2 pathogen assays with antibodies in patients. LFIA tests usually take around 10–30 min, while the conventional ELISA takes approximately 2–5 h. The sensitivity of COVID-19 detection by LFIA ranges from 61% to 88% (10 days after the first onset of symptoms) to 100% (after 3 weeks) [22][23]. However, early detection of the disease is a real challenge for LFIA, due to its low accuracy in detection. The accuracy of an LFIA device is evaluated in terms of its sensitivity and specificity. Thus, many efforts have been made to achieve higher sensitivity and specificity for SARS-CoV-2 detection in order to reduce false negative/positive predictive results. In a recent report, Xiang et al. showed that redesigned LFIA can obtain comparable sensitivity to ELISA [24]. Similarly, Smith et al. evaluated the sensitivity of the Quidel SARS Sofia rapid antigen flow immunoassay (USA) against RT-qPCR [25]. All tests achieved higher than 98% sensitivity to detect infected patients if tests were administered every three days. These evaluations confirmed the possibility of developing an ultrasensitive, highly specific LFIA for POC testing.
| Type | Test Kit | Sample Type | Biomarker | Detection Method | Sensitivity | Test Time | Characteristics |
|---|---|---|---|---|---|---|---|
| Antigen detection | BinaxNOW COVID-19 Ag Card, Abbott Diagnostics Scarborough, Inc. [40] | Nasal swab | N protein | Visual | 22.5 TCID50/swab | 15 min | POC testing; performance depends on following careful testing instructions |
| CareStart COVID-19 Antigen test, Access Bio, Inc. [41] | Nasopharyngeal Swab | N protein | Visual | 8 × 102 TCID50/mL | 10 min | Requires sample preparation step; operated by trained personnel | |
| Lumira Dx SARS-CoV-2 Ag Test, Lumira Dx UK Ltd. [42] | Nasal swab | N protein | Fluorescence | 32 TCID50/mL | 12 min | Requires Lumira Dx Platform; operated by trained personnel | |
| Sofia 2 Flu + SARS Antigen Flow Immunoassay, Quidel Corporation [43] |
Nasal, Nasopharyngeal swabs |
N protein | Fluorescence | 4.17 × 105 TCID50/mL | 15 min | Detection of SARS-CoV-2, Influenza A Virus, and Influenza B Virus; limited to Sofia 2 Instrument; operated by trained personnel | |
| Antibodies detection | Biohit SARS-CoV-2 IgM/IgG Antibody Test Kit, Biohit Healthcare (Hefei) Co., Ltd. [44] | Serum, plasma, venous whole blood (heparin, EDTA, and sodium citrate) | IgM and IgG | Visual | 96.7% | 10–20 min | Operated by trained personnel |
| COVID-19 IgG/IgM Rapid Test Cassette, Healgen Scientific LLC [45] | Serum, plasma, whole blood | IgM and IgG | Visual | 100% | 10 min | Operated by trained personnel | |
| Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2), Zhuhai Livzon Diagnostics Inc. [46] | Serum, plasma, venous whole blood | IgM and IgG | Visual | 90.6% | 15 min | - | |
| qSARS-CoV-2 IgG/IgM Rapid Test, Cellex Inc. [47] | Serum, plasma (EDTA or citrate), venous whole blood | IgM and IgG | Visual | - | 15 min | Operated by trained personnel | |
| Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette, Salofa Oy [48] | Serum, plasma, fingerstick whole blood | IgM and IgG | Visual | 93.3% | 15–20 min | Operated by trained personnel | |
| SARS-CoV-2 IgG IgM Antibody Rapid Test Kit, Lumigenex Co., Ltd. [49] | Serum, plasma, fingerstick whole blood | IgM and IgG | Visual | 100% | 15 min | Operated by trained personnel | |
| SARS-CoV-2 Antibody Test, Guangzhou Wondfo Biotech Co., Ltd. [50] | Serum, plasma, whole blood | IgM and IgG | Visual | 86.4% | 15 min | - | |
| RapCov Rapid COVID-19 Test, ADVAITE, Inc. [51] | Fingerstick whole blood | IgG | Visual | 90% | 15 min | Operated by trained personnel | |
| Rapid COVID-19 IgM/IgG Combo Test Kit, Megna Health, Inc. [52] | Serum, acid citrate dextrose plasma, fingerstick whole blood | IgM and IgG | Visual | 100% | 10–20 min | Operated by trained personnel |
In a comparison study, the average sensitivity of commercial kits (≈65%) was lower than the sensitivity of laboratory-based kits (≈88%) and of other serological methods (>80%) [53]. Moreover, although the claimed sensitivity and specificity of some commercial kits are high, the clinical accuracy of COVID-19 diagnosis is much lower, with the positive predictive value ranging from 11% to 50% [54]. In addition, LFIA detection devices are also associated with other challenges relating to difficulties controlling the fluid velocity and capillary force; the interferent porous membrane; the analysis time; and the sample nature [55][56]. Therefore, further efforts are needed to enhance the sensitivity and specificity of LFIA and ensure LFIA’s practical application in disease control and surveillance.
During the COVID-19 pandemic and beyond, the need for low-cost, simple, rapid, and highly accurate methods of disease detection is urgent. However, false negative and false positive test results due to low sensitivity and specificity make it difficult for lateral flow devices to detect disease at its early stages. Therefore, results of lateral flow devices are required to be confirmed with RT-PCR, in order to inform decision-making surrounding isolation and treatment. Up to now, many efforts have been made to enhance sensitivity and specificity of lateral flow technologies. Several methods have been developed, such as sample pre-concentration and amplification, signal enhancement using nanoparticles or an external signal reader, optimizing assay time, and the use of high affinity agents. A summary of methods for enhancing the sensitivity and specificity of lateral flow assays can be found in Table 2.
| Type | Test Kit | Sample Type | Biomarker | Detection Method | Sensitivity | Test Time | Characteristics |
|---|---|---|---|---|---|---|---|
| Antigen detection | BinaxNOW COVID-19 Ag Card, Abbott Diagnostics Scarborough, Inc. [40] | Nasal swab | N protein | Visual | 22.5 TCID50/swab | 15 min | POC testing; performance depends on following careful testing instructions |
| CareStart COVID-19 Antigen test, Access Bio, Inc. [41] | Nasopharyngeal Swab | N protein | Visual | 8 × 102 TCID50/mL | 10 min | Requires sample preparation step; operated by trained personnel | |
| Lumira Dx SARS-CoV-2 Ag Test, Lumira Dx UK Ltd. [42] | Nasal swab | N protein | Fluorescence | 32 TCID50/mL | 12 min | Requires Lumira Dx Platform; operated by trained personnel | |
| Sofia 2 Flu + SARS Antigen Flow Immunoassay, Quidel Corporation [43] |
Nasal, Nasopharyngeal swabs |
N protein | Fluorescence | 4.17 × 105 TCID50/mL | 15 min | Detection of SARS-CoV-2, Influenza A Virus, and Influenza B Virus; limited to Sofia 2 Instrument; operated by trained personnel | |
| Antibodies detection | Biohit SARS-CoV-2 IgM/IgG Antibody Test Kit, Biohit Healthcare (Hefei) Co., Ltd. [44] | Serum, plasma, venous whole blood (heparin, EDTA, and sodium citrate) | IgM and IgG | Visual | 96.7% | 10–20 min | Operated by trained personnel |
| COVID-19 IgG/IgM Rapid Test Cassette, Healgen Scientific LLC [45] | Serum, plasma, whole blood | IgM and IgG | Visual | 100% | 10 min | Operated by trained personnel | |
| Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2), Zhuhai Livzon Diagnostics Inc. [46] | Serum, plasma, venous whole blood | IgM and IgG | Visual | 90.6% | 15 min | - | |
| qSARS-CoV-2 IgG/IgM Rapid Test, Cellex Inc. [47] | Serum, plasma (EDTA or citrate), venous whole blood | IgM and IgG | Visual | - | 15 min | Operated by trained personnel | |
| Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette, Salofa Oy [48] | Serum, plasma, fingerstick whole blood | IgM and IgG | Visual | 93.3% | 15–20 min | Operated by trained personnel | |
| SARS-CoV-2 IgG IgM Antibody Rapid Test Kit, Lumigenex Co., Ltd. [49] | Serum, plasma, fingerstick whole blood | IgM and IgG | Visual | 100% | 15 min | Operated by trained personnel | |
| SARS-CoV-2 Antibody Test, Guangzhou Wondfo Biotech Co., Ltd. [50] | Serum, plasma, whole blood | IgM and IgG | Visual | 86.4% | 15 min | - | |
| RapCov Rapid COVID-19 Test, ADVAITE, Inc. [51] | Fingerstick whole blood | IgG | Visual | 90% | 15 min | Operated by trained personnel | |
| Rapid COVID-19 IgM/IgG Combo Test Kit, Megna Health, Inc. [52] | Serum, acid citrate dextrose plasma, fingerstick whole blood | IgM and IgG | Visual | 100% | 10–20 min | Operated by trained personnel |
If the same quantity of sample is used, conventional lateral flow technologies only achieve an average sensitivity of 66%, which is much lower than other serological assays. However, pre-concentration of the sample, before it is processed with lateral flow test, can significantly improve the assay’s sensitivity. Sharma et al. have used a magnetic field to pre-concentrate the analytes from the sample matrices to achieve 10 times higher sensitivity [57]. In addition, the antigen–reporter complex can also be concentrated during the lateral flow assay running process by applying an electric field. The so-called isotachophoresis method allows for improved equilibrium binding and thus lowers the detection limit up to 400 times [58]. Pre-amplification is another excellent technique for increasing sensitivity. Amplifying DNA/RNA targeted samples by PCR prior to the lateral flow assay process can significantly boost lateral flow assay sensitivity up to RT-PCR’s sensitivity level [59]. However, the PCR amplification technique requires expensive instruments and well-trained personnel. Although sample enrichment methods can help increase sensitivity by ten to hundreds of times (even reaching ultrasensitivity), these methods still require additional equipment, extra preparation steps, or prolong the testing period, making it difficult for them to use for POC testing.
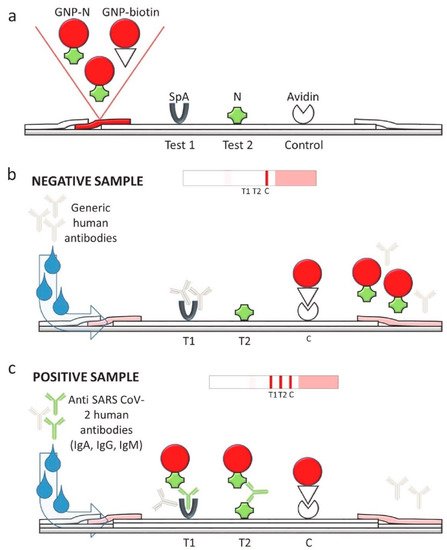
Specificity is another important factor that directly affects the accuracy of the lateral flow assay. Lateral flow assays for COVID-19 detection may be inaccurate due to the cross-reactivity of the SARS-CoV-2 virus with other coronaviruses. The cross-reactivity can reduce the specificity of the test, thus generating false positive results. To overcome this issue, phage display can be used to select SARS-CoV-2 antibodies with the strongest affinity. The phage display technique is a powerful method within the field of molecular biology that was awarded the 2018 Nobel Prize in Chemistry and has been widely used for the selection of antibodies, peptides, and disease-specific antigens [67]. In phage display, an exogenous DNA fragment encoding a protein of interest is inserted into a phage coat protein gene on the exposed surface, which is then capable of interacting with various external target molecules. This phenotype–genotype interaction enables researchers to isolate target-specific ligands [68].