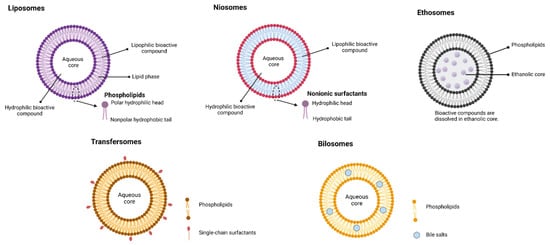
Liposomes vary with composition, size, surface charge, and method of preparation. The main attractive feature in the application of liposomes is that they can accommodate both water-soluble (in the aqueous core region) and lipid-soluble (in lamellae) bioactive compounds
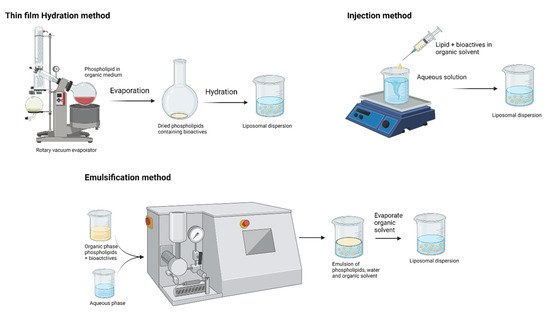
[32]. Schematic representations of liposome preparation techniques are shown in
Figure 4. These biodegradable and biocompatible compositions of liposomes make them excellent carriers of therapeutic agents. Conventional liposome preparation involves the following stages: drying of lipid/phospholipid ingredients from organic solvents, dispersion in aqueous media (it creates vesicles), purification of the resultant vesicles, and analysis of the final product. Thus, the increasing interest in lipid-based nutraceutical formulation can be correlated with the increasing number of clinical trials.
Table 2 summarizes the recent clinical trials on lipid-based formulations. However, the traces of organic solvent residues may remain in the lipid and/or aqueous phase and result in toxicity. Advances in the field of encapsulation technology have brought a possible way to prepare lipid vesicles without volatile organic solvents. It is a remarkable development by Prof. Mozafari to formulate microcapsules without the application of toxic solvents, detergents, and harsh treatments such as sonication/microfluidization
[38][39]:
3.1.1. Physicochemical Stability
The increasing interest in the liposomal delivery system is due to its functional properties, including efficient encapsulation capacity and biocompatibility with food constituents. However, the main problem associated with the industrial application of liposomes is their insufficient physiochemical stability due to the fragile bilayer phospholipid membranes and oxidation or hydrolysis of the fatty acids. One strategy to improve the physiochemical stability is the application of polysaccharides in liposomal preparation. The addition of polysaccharides (modifying surface charges) and antioxidants to liposomes minimizes the oxidative degradation of liposomes, including high-quality lecithins with low levels of hydroperoxides and transition metals
[43]. Lopes, et al.
[44] investigated the physicochemical properties and stability of the nisin (antibacterial agent) liposomes covered with polysaccharides (polygalacturonic acid/pectin). Polygalacturonic acid liposomes were stable for over 28 days, with a polydispersity index (PDI) of around 0.2 to 0.3, which indicates that the negatively charged polysaccharides increased the electrostatic repulsion between nanoparticles and thereby increased their stability. Later, the same group coencapsulated nisin and lysozyme in polysaccharide-coated liposomes (polygalacturonic acid/pectin). Polysaccharide-coated liposomes maintained constant particle and zeta potential due to the presence of polysaccharide layers on the surface or induced stabilization through the reduction of charges on the polysaccharide and liposome due to electrostatic screening
[45].
Azzi, et al.
[46] reported the higher encapsulation capacity and improved physicochemical properties of quercetin encapsulated with cyclodextrin-coated liposomes. Quercetin is a flavonoid known for its beneficial effects, including antiobesity, anticancer, anti-inflammatory, and antioxidant properties. However, poor water solubility and poor chemical stability are the main drawbacks of its application in the food sector. Due to poor aqueous solubility, quercetin possesses low bioavailability and a short biological half-life
[47]. The authors revealed that the quercetin-loaded liposomes were stable (evaluated through particle size and quercetin content) even after 12 months of storage (@ 4 °C). Surprisingly, a higher amount of quercetin (98%) was retained after 12 months in the quercetin in cyclodextrin in liposomes. Pu, et al.
[48] came up with a different strategy with cationic guar gum (CGG) to increase the stability of curcumin. CGG is the cationic quaternary group of guar gum (GG) and has a good affinity for negatively charged compounds. As a coating material, nonionic GG might coat the negatively charged liposomes with physical absorption, but the cationic CGG is absorbed on the negative surface of the liposomes via electrostatic interaction and remains stable. The negative charge of the liposomal surface was neutralized by the CGG interactions and turned the net charge positive. The authors observed the better protection effect (able to provide higher curcumin retention at 70 °C) of curcumin-loaded liposomes coated with CGG. On the other hand, nanoliposomes have been reported as a promising delivery system for transporting food bioactive compounds.
Plant-based phospholipids (PLs), such as soybean, rapeseed, sunflower PLs, were frequently used for liposomal preparation, and the major PL components include zwitterionic phosphatidylcholine (PC), phosphatidylethanolamine (PE), and anionic phosphatidylinositol (PI). However, those plant-based PLs are more highly susceptible to oxidation and lower physical and storage stability owing to the high degree of unsaturation
[49]. Alternatively, animal-based PLs, such as eggs, milk, meat, and marine source, are gaining attention for the fabrication of liposomes. Interestingly, marine PLs (fish roe, krill oil, and fish) are reported for higher PUFAs, which makes them a promising functional ingredient
[50]. Milk PLs contain high levels of sphingomyelin (SM) in addition to other PL components and are reported to have a higher degree of saturation. Wu, Mou, Song, Tan, Lai, Shen and Cheong
[49] studied the effect of bovine milk and krill PLs for curcumin-loaded liposomes and noticed that the liposome of bovine milk PLs exhibited higher stability with a smaller particle size and greater zeta potential (163 nm and −26.7 mV) than the krill PL liposomes (212 nm and −15.23 mV). Both curcumin liposomes were stable (curcumin retention: 80%) after 40 h exposure in acidic conditions, but the retention rate of curcumin was reduced to 50% at neutral and alkaline conditions. Additionally, curcumin retention is higher in bovine milk PL formulation than the krill PLs attributed to the high amount of phosphatidylcholine in krill PLs, which are susceptible to hydrolysis, resulting in the leakage of curcumin from the phospholipids.
3.1.2. Enhancing the Bioavailability of Polyphenols by Liposomal Technology
There is always a demand for plant-derived bioactive compounds, which are considered natural and safer. Polyphenols are the secondary plant metabolites, which include nearly 10,000 different compounds with one or more aromatic rings and hydroxyl groups. Readers can refer to Brglez Mojzer, et al.
[51] for an understanding of the polyphenols and the different extraction methods. Polyphenols are generally classified as flavonoids and phenolic acids. Additionally, flavonoids are further divided into flavones, flavonols, flavanones, and isoflavones, and phenolic acids as hydroxybenzoic and hydroxycinnamic acids
[52]. However, the poor absorption and low bioavailability of extracted polyphenols restrict the wide application of polyphenol-based functional foods. Entrapping those polyphenols in the bilayered vesicular systems (liposomes) is one of the ideal ways to improve GI stability and bioavailability.
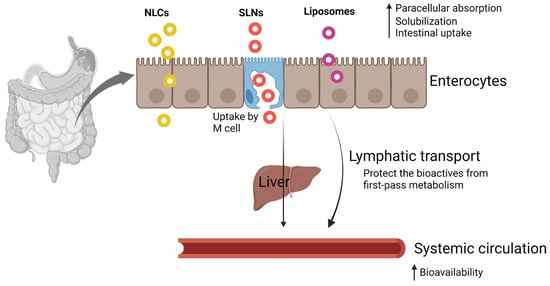
However, conventional liposomes or nanoparticles do not cross the intestinal mucosal barrier due to their relatively large size
[53], are unstable in the gastric environment, and release the entrapped bioactive compounds prior to the target site. From the previous research, it is evident that the bioactive compounds need to be absorbed/transformed into mixed micelles in the GI tract and transported to the epithelial cells. M-cell-mediated transport is reported for the possible absorption route for liposomes and nanoparticles; however, the M-cells may account for 1% of the total epithelial cell population
[54]. The following strategies were employed for enhancing the intestinal absorption of encapsulated bioactive compounds.
Curcumin: Curcumin is a polyphenolic compound reported for its antioxidant, anti-inflammatory, antimicrobial, and anticancer properties. However, the wide application of curcumin is limited because of its poor aqueous solubility, rapid metabolism, and low permeability due to its susceptibility to P-glycoprotein efflux
[55]. The stability of curcumin in the liposomes can be improved further by converting the liquid formulation into a dry form. Additionally, conventionally either spray drying or freeze drying is used for this purpose. Alternatively, Gopi, et al.
[56] prepared liposomal curcuminoids in powder form through a novel nanofiber weaving technology. The release of curcumin from the liposomal curcuminoid powder was evaluated at pH 5.5 (46.2%) and pH 7.4 (40.4%), and the burst release (initial rupture) was observed within 12 h and then followed a slow and sustained release. A slightly acidic pH accelerated the release of curcumin from the formulation, and the strong interaction between phospholipids and curcumin at pH 7.4 showed less desorption.
Recent research on Pluronic-modified liposomes showed promising results in maintaining the stability of bioactives in the GI tract and improving bioaccessibility. Pluronics (also called poloxamer, Kolliphor, and Synperonic) are nonionic triblock copolymers with a central hydrophobic poly (propylene oxide (PPO)) chain with two hydrophilic poly (ethylene oxide (PEO)) on each side
[57]. Pluronic-modified liposomes protected the curcumin from degradation, and nearly 50% of curcumin was retained after exposure to 80 °C. Additionally, the bioaccessibility of curcumin was increased to 43.3%, whereas the simple curcumin liposomal formulation was shown to be 26.9% bioaccessible
[58]. Further, the same group revealed Fluronic-modified liposomes, and folated Fluronic-modified liposomes are nontoxic to the human KB cell lines
[59].
Green tea polyphenols: Green tea comprises more than eight polyphenolic compounds (catechins) and is highly interesting among consumers for its potential health benefits, including antioxidant, antidiabetic, anti-inflammation properties. However, catechins are poorly absorbed in the body. When consuming green tea, only 5% of tea catechins reached the systemic circulation in rats and 1.68% in humans
[60]. Liposomal formulation of catechin improves the GI stability and absorption in the body. Ezzat, et al.
[61] developed chitosan-coated catechin liposomes and evaluated the pharmacokinetic properties in Wistar rats. Intestinal permeation from the in situ intestinal perfusion study revealed the rapid increase in intestinal absorption of chitosan-coated catechin liposomes (45.80 µg in 30 min); at the same time, conventional liposomal formulation and catechin solution absorbed only 18.26 and 7.36 µg, respectively. Similarly, the bioavailability of chitosan-coated catechin liposome was 1.37-folds higher than that of conventional liposomes and 2.12-folds higher than that of the catechin solution. Therefore, chitosan can directly interact with the tight junctions and facilitates the paracellular transportation and makes the chitosan-entrapped liposomes a promising approach for improving the intestinal absorption of bioactive compounds. Researchers worked with the different strategies to improve the bioavailability of catechins, including nanoencapsulation of catechins and nanoencapsulated catechin-based functional foods, and on different routes of delivering catechin. Recently, Fornasier, et al.
[62] formulated catechin hexosomes (lipids aggregated in tubular arrangement) for the topical administration and found increasing penetration of catechins in the pig skin. To enhance the permeation properties, bile salts were used in the formulation and found that hexasomes with bile salts showed deeper transdermal penetration of the catechins than the hexasomes without bile salts.
Resveratrol: Resveratrol (trans-resveratrol; 3,5,4′-trihydroxystilbene) is a nonflavonoid polyphenolic compound found in grapes, red wine, and berries. Resveratrol is known for its health benefits, including anti-inflammatory, antiobesity, antioxidant, anticarcinogenic, and antiaging properties. On the downside, resveratrol is photosensitive and poorly soluble at the low GI phase and has low intestinal absorption and rapid metabolism. To overcome these limitations, nanosized formulations including liposomal entrapment of resveratrol can be employed. The liposomal formulation of resveratrol is gaining attention for its anticancer properties. A combination of anticancer bioactive compounds, resveratrol, and artemisinin in liposomes was evaluated for intestinal cancer cells
[63]. The authors found that Eudragit-coated liposomes of both resveratrol and artemisinin reduced the viability of the HT-29 cells to 16% and 37% after 24 h exposure to 10 and 20 µg mL
−1, respectively. The increased mortality of tumor cells was attributed to resveratrol’s multiple cellular targets affecting cellular proliferation and growth, including apoptosis, inflammation, invasion, angiogenesis, and metastasis
[64].
Proliposomes: Proliposomes are a solidified form of liposomes formulated by removing water content in the liposomal suspension using different techniques: spray drying, freeze drying, vacuum drying, and fluidized bed drying. These dry granular proliposomes do not form a lipid bilayer structure during storing (increases stability), and they can be converted to liposomal formulation upon hydration. In a recent study, Jiao, et al.
[65] optimized the supercritical fluid (ScCO
2) technique for vitamin C (VC) proliposomes and reported that the optimal conditions were 25 MPa as pressure, 48 °C as temperature, and 0.25 as feeding ratio of vitamin C against phosphatidylcholine. This solid-state formulation ensures stability and is convenient for transportation, storage, and distribution. The solubility of proliposomes loaded with curcumin is estimated to be 98%, indicating the outstanding dispersible characteristics of proliposomes at the hydration step
[66]. Further, proliposomes maintained an encapsulation efficiency of 92% after 30 days of storage at room temperature
[67]. An in situ intestinal absorption study proved the increase in the absorption of liposomal formulations compared with the free form. Ren, et al.
[68] reported a significant increase in absorption rate constant (2.3-fold) and absorptive fraction (1.4-fold) between the proliposome dispersion and its free form (quercetin was the model bioactive compound). Surprisingly, the in vivo pharmacokinetic study revealed a nearly 6.5-fold increase in C
max (maximum concentration) and a 12-fold increase in AUC (area under the curve) for the quercetin proliposomes. Such drastic increase in oral bioavailability was attributed to the solid formulation of quercetin proliposomes and their stability during GI transit
[69]. Recently, Hızır-Kadı, et al.
[70] improved the solubility of pollen phenolic extract (PPE) and improved the bioaccessibility by 2-fold compared with the conventional liposomes. However, the authors dissolved PPE in a few drops of ethanol during the proliposome production step, which may restrict the wide application of following this technique in an industrial scale.
Bile salt liposomes: To extend the application of liposomes further, bile salts are incorporated into the encapsulation process of liposomes, and such bile-salt-incorporated liposomes are called ‘nanobilosomes’. Bile salts in the ‘nanobilosomes’ further improve the aqueous solubility and dissolution rate and preserve the liposomes in the GI tract and enhance membrane permeability
[71]. This ‘nanobilosome’ can be a promising technique to deliver the Biopharmaceutics Classification System (BCS) class IV bioactive compounds, which exhibit poor solubility and poor intestinal permeability. Mangiferin polyphenol is one of the poorly aqueous soluble (0.162 mg/mL solubility in water) and poorly intestinally permeable compounds (1.96 effective permeability in duodenum)
[72]. Nanobilosome formulation improved the aqueous solubility by seven times and had better protection in the simulated gastrointestinal condition due to the electrostatic repulsive force between the bile salts in the formulation and the bile salts in the simulated intestinal fluid
[73].