2. Analysis on Results
2.1. Survival Rate of Islets
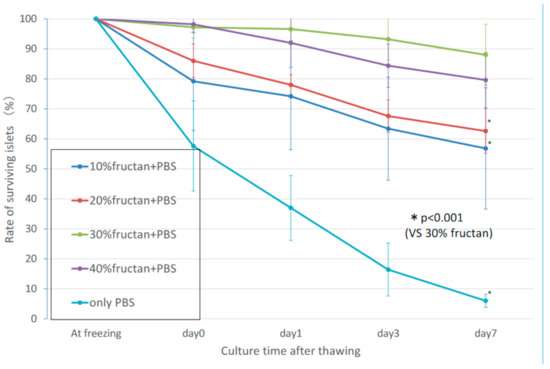
In the fructan groups, the rate of decrease in the numbers of thawed cultured pancreatic islets over time reduced with increasing fructan concentration. Concentrations of 30% fructan or higher resulted in a favorable survival rate, with 95% or more of the pancreatic islets showing a maintained morphology on day 3. (Figure 1). There was no significant difference in islet survival between the 30% and 40% fructan groups (p = 0.059), but the 30% fructan group showed significantly higher survival than the 20% fructan group (p < 0.001). Furthermore, the 10% DMSO group showed a survival rate of approximately 95% at thawing, and the number of islets decreased on approximately 65% on day 3. Therefore, the 30% fructan group showed significantly higher survival compared to the 10% DMSO group (p < 0.001).
Figure 1. Number of surviving islets in the fructan groups after cryopreservation.
2.2. Morphology
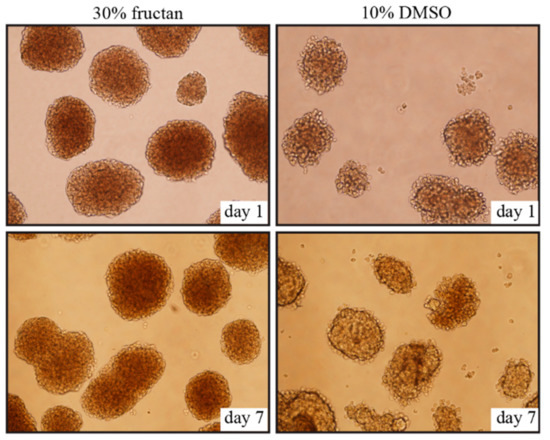
No significant changes in morphology were observed from day 1 to day 7 after thawing, and cell clusters of pancreatic islets were well-maintained in the 30% fructan group; however, cell cluster margins collapsed and vacuolation was found in the interiors of islets in the DMSO groups (Figure 2).
Figure 2. Morphology of the islets cryopreserved in 30% fructan or 10% DMSO after 1 day and 7 days.
2.3. Insulin Release Assay
In all of the fructan groups, the insulin secretion volume in 3.3 mM and 20 mM of glucose media was significantly higher than that in the DMSO groups. Furthermore, the 30% fructan group showed the highest stimulation index of all fructan groups at 2.65, and the 10% DMSO group showed almost the highest S.I of all DMSO group at 3.13. The difference in the stimulation index were not observed in all of the group (Table 1).
Table 1. Insulin release assay.
Insulin Release
(n = 6) |
Low1
(ng/mL/h) |
Hi
(ng/mL/h) |
Low2
(ng/mL/h) |
S.I |
| 10%fructan + PBS |
7.19 ± 1.13 |
16.54 ± 2.05 |
7.13 ± 1.31 |
2.32 ± 0.35 |
| 20%fructan + PBS |
7.03 ± 2.81 |
14.00 ± 3.38 |
7.61 ± 3.08 |
2.45 ± 1.00 |
| 30%fructan + PBS |
6.81 ± 1.41 * |
17.97 ± 5.15 * |
6.98 ± 1.35 * |
2.65 ± 0.62 |
| 40%fructan + PBS |
5.74 ± 1.90 |
13.17 ± 1.86 |
6.07 ± 2.61 |
2.55 ± 0.95 |
| 2.5%DMSO + FBS |
5.59 ± 4.90 |
8.97 ± 4.38 |
4.31 ± 2.26 |
2.32 ± 1.48 |
| 5.0%DMSO + FBS |
3.95 ± 1.09 |
8.77 ± 2.38 |
3.91 ± 1.44 |
2.43 ± 1.14 |
| 7.5%DMSO + FBS |
4.85 ± 2.18 |
9.49 ± 2.23 |
4.84 ± 2.65 |
2.25 ± 0.72 |
| 10%DMSO + FBS |
3.66 ± 2.61 |
9.93 ± 4.26 |
4.18 ± 2.78 |
3.13 ± 0.76 |
2.4. Islet Transplantation
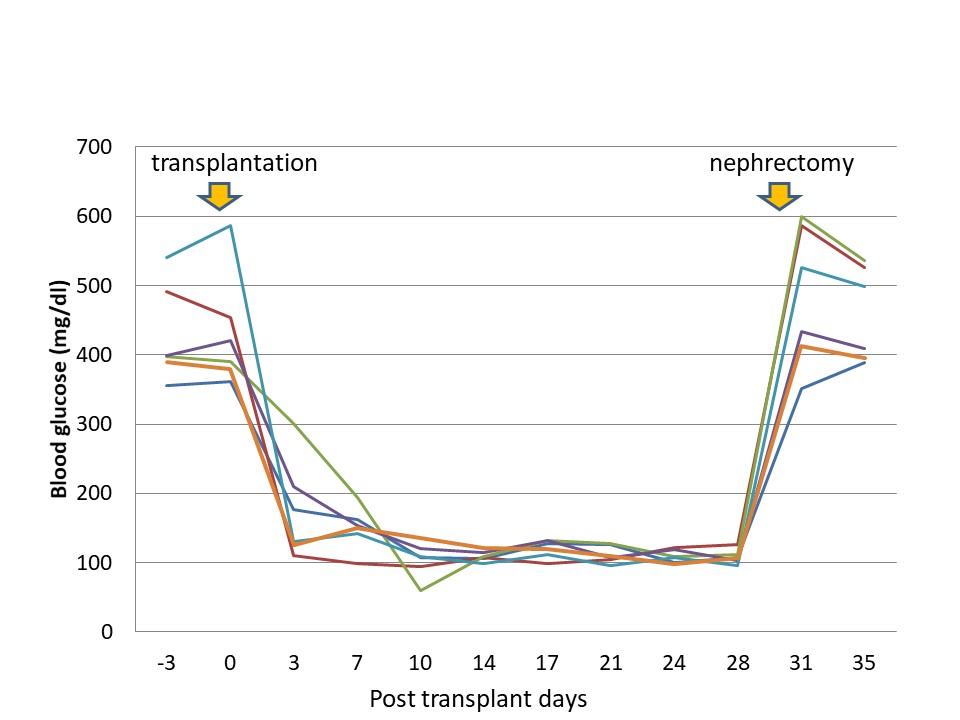
Approximately 800 pancreatic islets frozen with 30% fructan were transplanted to streptozotocin-induced diabetic rats at the first day after thawing (n = 6). The blood glucose levels in all diabetic rats fell to within the normal range within one week after transplantation, and was stable thereafter. After removal of the kidneys along with transplanted pancreatic islets, blood glucose levels rose again (Figure 3). No adverse events were found in any case.
Figure 3. Effects of islet transplantation on blood glucose levels in diabetic rat (n = 6).
2.5. Histology
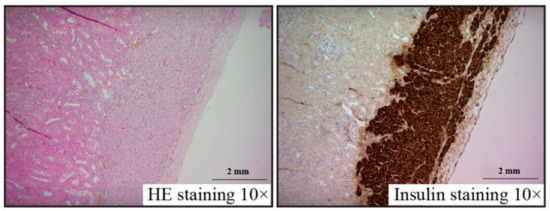
Pancreatic islet renal grafts frozen with 30% fructan were observed under a microscope with HE and insulin staining. Survival was confirmed with no abnormalities within the renal capsule, and insulin staining was positive in all specimens (Figure 4). No differences in morphology were found compared to sections of unfrozen pancreatic islets implanted within renal capsules.
Figure 4. Islet graft histology at 28 days after transplantation.
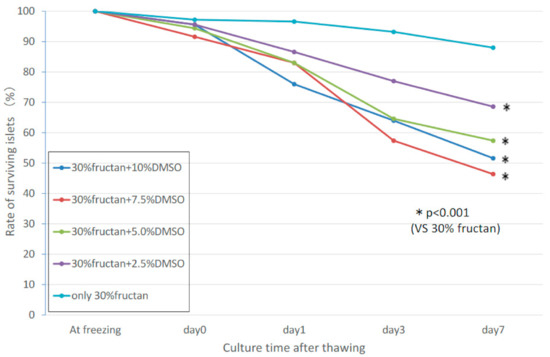
2.6. Synergistic Effects of Fructan and DMSO
Based on the results of the fructan-only cryopreservation liquid and FBS + DMSO cryopreservation liquid, we evaluated the possibility of synergistic effects in a cryopreservation liquid containing both DMSO and fructan, using various concentrations of DMSO (2.5%, 5.0%, 7.5%, 10.0%) and 30% fructan solution. Islets in the 30% fructan-only group showed significantly higher survival compared to all of the DMSO-added groups, and no synergistic effects were found (Figure 5). The insulin release assay showed that the stimulation index of the fructan + DMSO groups was greater than that of the fructan-only groups, but the fructan group had a higher insulin secretion volume than the fructan + DMSO groups with both 3.3 mM and 20.0 mM of glucose medium (Table 2).
Figure 5. Synergistic effects of fructan and DMSO on islet survival rate during cryopreservation.
Table 2. Study of synergistic effects of fructan and DMSO Insulin release assay.
Insulin Release
(n = 6) |
Low1
(ng/mL/h) |
Hi
(ng/mL/h) |
Low2
(ng/mL/h) |
S.I |
| 30%fructan + PBS |
6.81 ± 1.41 * |
17.97 ± 5.15 * |
6.98 ± 1.35 * |
2.65 ± 0.62 |
| 10%DMSO + FBS |
3.66 ± 2.61 |
9.93 ± 4.26 |
4.18 ± 2.78 |
3.13 ± 0.76 |
| 30%fructan + 10%DMSO |
3.81 ± 1.48 |
11.86 ± 5.85 |
3.30 ± 2.02 |
3.11 ± 0.90 |
| 30%fructan + 7.5%DMSO |
4.91 ± 3.57 |
12.58 ± 7.58 |
4.98 ± 3.70 |
2.92 ± 1.80 |
| 30%fructan + 5.0%DMSO |
5.09 ± 1.53 |
11.59 ± 5.04 |
5.04 ± 1.78 |
2.25 ± 0.68 |
| 30%fructan + 2.5%DMSO |
4.31 ± 2.83 |
12.10 ± 6.30 |
4.80 ± 3.75 |
3.31 ± 1.12 |
3. Current Insights
Pancreatic islet transplantation is performed as a therapeutic method for type 1 diabetes mellitus mainly in Europe and the USA. A five-year follow-up survey of patients receiving pancreatic islet-only transplantation showed that C-peptides were maintained in approximately 80% of cases; however, the non-insulin dependency rate dropped to 71% after one year, 52% after two years, and 23% after three years, with approximately 90% of cases becoming insulin-dependent again after five years
[2]. Therefore, multiple pancreatic islet transplantations may be required to achieve a favorable outcome and improve quality of life. However, pancreatic islets are often not readily available owing to a lack of donors, or transplants cannot be performed because of an insufficient number of pancreatic islets from a single donor. Therefore, development of a technique that could limit the fixed amount of pancreatic islets lost from cryopreservation and maintain pancreatic islet function would be useful in eliminating the future shortfalls in pancreatic islets and help to establish an islet bank.
Several methods for the cryopreservation of cultured cells have been developed and tested, with emphasis placed on using cryoprotectants that are highly water-soluble, allowing for a high degree of concentration during freezing, and have no cytotoxicity
[3][4]. DMSO is commonly used as a low-molecular-weight cryoprotectant. It easily penetrates cell membranes, and is exemplified by ethylene glycol, trimethylene glycol, methanol, dimethylacetoamide, and glycerol, which each demonstrate cryoprotective action
[5]. By contrast, high-molecular-weight cryoprotectants, such as polyethylene glycol, polyvinylpyrolidon, hydroxylethyl starch, dextran, and albumin, show cryoprotective action that stops outside of cells
[5][6]. Of these cryoprotectants, glycerol is frequently used as a substitute for DMSO to preserve red blood cells at a concentration of 10%, although DMSO is clearly superior with respect to cell survival rate
[7][8]. However, DMSO also shows strong cytotoxicity, and in addition to being used as a cryoprotectant, it is also used as a differentiation inducing agent for cultured cells. Therefore, the use of DMSO is not recommended when aiming for stability during cell storage
[9][10][11]. In addition, serum is often used during cryopreservation with DMSO, which poses another problem. When animal serum is used, there is a concern of zootic infection such as bovine spongiform encephalopathy
[12]. In addition to its high cost, these disadvantages have motivated the development of a serum-free cryopreservation liquid. Fructan as a natural plant compound shows good potential in this regard. It is assumed that fructan in plants may serve as an anti-freezing agent (cold protectant), as well as an osmotic protectant (protection against dryness or acid) or other protectant against drought. Furthermore, fructan is known for its cell membrane strengthening, stabilization action, anti-allergy effects, and anti-tumor activity
[11][13]. In particular, Graminan-type fructan is a polysaccharide stored in plants such as
rakkyo (Japanese leek) and wheat, and various activities are expected since it has a highly branched structure
[14]. The
rakkyo fructan used in this experiment was graminan-type, which links at a 3:1 ratio of beta-2,1 linking to beta-2,6 linking. It is characterized by the fact that it is easily dissolved in cold water (30% or more solubility in 4 °C water) and is capable of high-pressure steam sterilization; Ogawa et al.
[15] demonstrated its efficacy as a mammalian cell cryoprotectant. The key factors contributing to this effect are that it is highly water-soluble and can be highly concentrated during freezing, and that it prevents cell membrane damage from ice crystal formation on the outside of cells by enveloping the membranes like a net
[15].
In our study, rakkyo fructan was used to determine its potential application as a pancreatic islet preservation liquid using rat pancreatic islets. First, the optimum concentration was determined by comparing survival rates in order to apply fructan to the cryopreservation of pancreatic islets. Fructan concentration-dependent improvement in survival rate was observed, with significantly higher survival at concentrations of 30% or less (p < 0.05).
The survival rate immediately after thawing in the 30% fructan group was 97.2 ± 3.6%, and survival decreased gradually but was maintained above 93% after 3 days. Between fructan concentrations of 30% and 40%, the 30% group showed signs of a higher pancreatic islet survival rate, but no significant difference was found. Moreover, 30% is thought to be the optimum concentration for cryopreservation of pancreatic islets with fructan, i.e., the result of ruling out toxicity to pancreatic islets even at high concentrations of fructan.
Next, we compared the effects of 30% fructan (considered to be the optimum concentration) with those of 10% DMSO solution (the commonly used cryopreservation liquid). Although there was no difference in the survival rate between the two groups immediately after thawing, there was a sharp decrease in survival rate for the 10% DMSO group, reaching 64.8 ± 14.6 by the third day. Morphological observations of cultured pancreatic islets after thawing showed that islet cell clusters in the fructan groups were well-maintained even after 3 days, whereas the cell cluster margins had collapsed and vacuolation was found in the islets in the DMSO groups. As mentioned above, fructan is considered to demonstrate a protective effect against the structural collapse of cells due to cold stress by stabilizing the cell membranes, but its action on pancreatic islets (which are cell clusters) is assumed to be due to protection from external stimulation (for islets, not individual cells). On the other hand, although DMSO is commonly used as a cryoprotectant to protect cells from ice crystal formation due to freezing on the inside and outside of cells, it ultimately decreased the survival rate, which is likely a result of penetrating the cell clusters in pancreatic islets leading to their collapse. There was no synergistic effect of the fructan-only group. In addition, addition of the low (2.5%) DMSO concentration to fructan resulted in better survival of pancreatic islets compared to the high (10%) DMSO concentration. This suggests that the cytotoxic effects of DMSO outweigh its benefits as a freezing agent for rat pancreatic islets.
The insulin release assay of pancreatic islet function showed that the stimulation index exceeded 2 in both the 30% fructan group and the DMSO groups, suggesting that the beta cells that escaped apoptosis from the freezing and thawing process were still capable of glucose concentration-dependent insulin secretion. Although there was no difference in the stimulation index between the DMSO and fructan groups, the insulin secretion volume at each glucose concentration was significantly higher in the fructan groups, which might reflect the difference in pancreatic islet survival rates (beta cell survival rate).
After pancreatic islets cryopreserved with 30% fructan were transplanted to diabetic rats, individual glucose levels were normalized in all cases and a stable blood glucose level was achieved. Furthermore, the survival of pancreatic islets transplanted to renal capsules was confirmed microscopically. No notable adverse events were found in rats, even when the low-concentration fructan (0.1%) + 3 mL PBS solution was intravenously injected into the rat tail veins. Although there is potential that fructan might be harmful to the living body, this has not yet been completely ruled out, as data on survival rates suggests that it could be considered safe.