2. Intraocular Pressure
Elevated intraocular pressure represents the only currently approved modifiable risk factor for POAG. While the exact molecular mechanisms underlying elevated IOP and its relationship to glaucoma are not fully understood, it is well known that elevated IOP is strongly associated with both structural and functional glaucomatous change
[3][11]. Currently, this relationship is described by mechanical strain that elevated IOP places on the lamina cribrosa and adjacent optic nerve tissues, ultimately resulting in structural damage that disrupts axonal transport
[3][12]. It has also been suggested that there may be a pressure-induced metabolic stress effect, and even circulatory, immune, and oxidative stress mechanisms associated with glaucoma and related to IOP
[3][13][14].
Interestingly, elevated IOP has shown a relatively strong heritability estimate. When Asefa et al. conducted a systematic review and meta-analysis for the heritability of glaucoma, they also evaluated pooled h
2 estimates for endophenotypes, including IOP, which they estimated at h
2 = 0.43 (95% confidence interval (CI) 0.38–0.48). Importantly, in their analysis, IOP was the most commonly reported endophenotype, reported in 41 different studies. This large sample also allowed for subgroup analysis, showing that there was heterogeneity between age and ethnicity. Findings showing different heritability estimates between White Europeans (h
2 = 0.47, 95% CI 0.37–0.57,
p = 0.006), East Asians (h
2 = 0.49, 95% CI 0.40–0.59), and Other Populations (h
2 = 0.30, 95% CI 0.22–0.39) are particularly interesting given suspected genetic contributions to disease and genetic differences among different racial groups
[5][8]. Additionally, it should be noted that IOP has also shown a genetic correlation with POAG (odds ratio 1.18, 95% CI 1.14–1.21,
p = 1.8 × 10
−27)
[15].
To date, a wide variety of genes have been associated with elevated IOP, including, but not limited to:
ABCA1,
ABO,
ADAMTS8,
ADAMTS17,
ADAMTS18-NUDT7,
AFAP1,
ANGPT1,
ANTXR1,
ARHGEF12,
ARID5B,
ATXN2,
CAV1-CAV2,
CDKN2B-AS1,
CELF1,
CYP26A1-MYOF,
FAM125B,
FNDC3B,
FOXC1,
FOXP1,
GAS7,
GLCCI1-ICA1,
GLIS3,
GMDS,
HIVEP3,
INCA1,
LMX1B,
LOC171391,
MADD,
MIR548F3,
MYBPC3,
NDUFS3,
NR1H3,
PDDC1,
PKHD1,
PTPRJ,
RAPSN,
RPLP2-PNPLA2,
SIX1/SIX6,
SEPT9,
SP11,
TFEC-TES,
TMCO1, and
TXNRD2 [5][16]. It should be noted that all of these genes have not also been independently associated with POAG. Similarly, molecular mechanisms of these genes are generally not well understood (
Table 1).
Table 1. Selection of genes associated with IOP and suggested molecular mechanisms.
| Gene |
GWAS Identified |
Suggested Mechanism |
Reference |
| LMX1B |
Gao et al., 2018 [16] |
Altered Anterior Segment Development; Altered Aqueous Humor Dynamics |
Pressman et al., 2000 [17] |
| MADD |
Gao et al., 2018 [16] |
TNF-a-Mediated Microglial Activation |
Tezel et al., 2000 [18] |
| NR1H3 |
Gao et al., 2018 [16] |
Alteration of IOP via-ABCA1-Regulated Aqueous Humor Dynamic Alterations |
Hu et al., 2020 [19] |
| SEPT9 |
Gao et al., 2018 [16] |
Cytoskeletal Alterations |
Ou et al., 1998 [20] |
In 2018, Gao et al. conducted the largest known GWAS for IOP using patients of European Descent (ED) from the UK Biobank (
n = 115,486). They identified 671 significant genetic variants associated with IOP, mapping to 149 loci, significantly expanding the known literature and replicating findings from previous studies. Importantly, they specifically identified four significant novel genes:
LMX1B,
MADD,
NR1H3, and
SEPT9 [16].
LMX1B is a gene that is directly implicated in the development of serotonergic (5-HT) neurons
[21]. According to Ding et al.,
LMX1B is a LIM homeodomain-containing gene, which they suggest is a key intermediate factor necessary for the cascade of specification and differentiation of 5-HT neurons
[21]. In mouse models, the LMX1B transcription factor has been associated with the regulation of anterior segment development, with
LMX1B transcripts being identified in the ciliary body and trabecular meshwork (
Table 2)
[17]. In fact, Pressman et al. described that mice with targeted
LMX1B mutations have structural deficiencies in the ciliary body, corneal stroma, and iris
[17]. If
LMX1B mutations are associated with alterations in the ciliary body and anterior chamber structures, it is logical to consider that aqueous humor dynamics may be altered, leading to elevated IOP and, potentially, POAG, though further evaluation is surely warranted (
Figure 1).
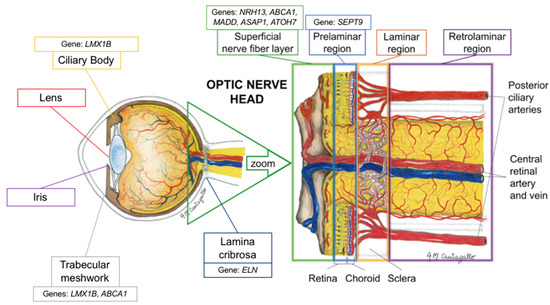
Figure 1. Schematic representation of the proposed location of the genes’ mechanism of action (adapted with permission from: Prada D, Harris A, Guidoboni G, Siesky B, Huang AM, Arciero J. Autoregulation and neurovascular coupling in the optic nerve head. Survey of Ophthalmology 1 March 2016; 61(2): 164–186).
Table 2. List of IOP and POAG genes under investigation.
| Gene |
Study |
Environment |
| ABCA1 |
Hu et al., 2020 [19] |
Ex vivo Human Model |
| ABCA1 |
Li et al., 2018 [22] |
In vivo Mouse Model |
| ABCA1 |
Yeghiazaryan et al., 2005 [23] |
In vivo Human Model |
| ATOH7 |
Song et al., 2015 [24] |
In vivo Rat Model |
| ATOH7 |
Miesfeld et al., 2020 [25] |
In vivo Mouse Model |
| ELN |
Gelman et al., 2010 [26] |
Ex vivo Mouse Model |
| LMX1B |
Cross et al., 2014 [27] |
In vivo Mouse Model |
| LMX1B |
Pressman et al., 2000 [17] |
In vivo Mouse Model |
| MADD |
Schievella et al., 1997 [28] |
In vitro Yeast Interaction Trap |
| NR1H3 |
Wang et al., 2002 [29] |
In vivo Mouse Model |
| NR1H3 |
Yang et al., 2014 [30] |
In vivo Mouse Model |
| NR1H3 |
Zheng et al., 2015 [31] |
In vivo Mouse Model |
| NR1H3 |
Song et al., 2019 [32] |
Ex vivo Mouse Model |
| SEPT9 |
Ghossoub et al., 2013 [33] |
Ex vivo Human Model |
| SEPT9 |
Ou et al., 1998 [20] |
In vivo Guinea Pig Model |
It should be noted that
LMX1B mutations have been associated with families affected by nail–patella syndrome (NPS), a rare autosomal dominant developmental disorder with variable presentation, and POAG, suggesting an explanation for the association between NPS and glaucoma (approximately 1/3 of patients with NPS develop glaucoma), though not explaining a mechanism for causation
[34][35][36]. In 2018, Choquet et al. suggested that the variability in glaucoma phenotypes associated with
LMX1B may be explained by different mutations. By inducing different
LMX1B mutations in a murine model, they found that different
LMX1B mutations can result in elevated IOP and glaucomatous damage independent of developmental defects, suggesting that
LMX1B is a significant glaucoma-susceptibility locus even in the absence of developmental abnormalities
[37]. Still, further research is necessary to better understand the role of
LMX1B in glaucoma pathogenesis.
NR1H3 is a nuclear receptor also referred to as the liver X receptor α. Liver X receptors regulate lipid homeostasis, with expression in both the liver and the brain, and play a key role neurodegenerative disease
[29]. According to Yang et al., liver X receptor α may also play an anti-inflammatory role, as demonstrated by a mouse model of autoimmune uveitis (
Table 2)
[30]. In fact, they suggested that liver X receptor α may inhibit the NF-kB signaling pathway, resulting in the reduction in ocular inflammation. These findings reflect those of Zheng at al., which suggest that the activation of liver X receptor β induces a protective effect on the retina perhaps by decreasing retinal amyloid β formation and by inhibiting the NF-kB signaling pathway
[31]. While Yang et al. investigated this anti-inflammatory effect in the context of autoimmune uveitis therapeutics, recent research has demonstrated the utility of the anti-inflammatory effect of liver X receptors in relation to glaucoma.
In 2019, Song et al. demonstrated that liver X receptors α and β were expressed in the retina and optic nerve (
Figure 1)
[32]. They also demonstrated that there was an accumulation of amyloid deposition in the retinal ganglion cells of liver X receptor β knockout mice, and that there was activation of microglia and loss of aquaporin 4 (a biomarker of optic nerve function) in the optic nerve. Ultimately, they reported that the loss of liver X receptor β results in retinal ganglion cell loss and optic nerve degeneration (
Table 2)
[32]. Interestingly, they noted that aquaporin 4 expression decreased without the presence of aquaporin 4 antibodies, suggesting that optic nerve degeneration in this model is caused by the loss of aquaporin 4 expression
[32]. While these findings are encouraging, they do not directly explain the association between
NR1H3 and IOP as suggested by the 2018 GWAS from Gao et al.
[16].
The association between
NR1H3 and IOP may actually be explained by
ABCA1, a POAG-susceptibility locus associated with ED populations as well as Asian populations
[5][38][39]. ABCA1, the ATP-binding cassette transporter A1, is a transmembrane transporter involved in cholesterol homeostasis through the formation of high-density lipoprotein
[40]. Importantly, ABCA1 is regulated by the liver X receptor
[41]. While ABCA1 has been implicated in retinal inflammation and retinal ganglion cell apoptosis, recent research has also demonstrated that ABCA1 has a role in regulating IOP through aqueous humor dynamics
[19][22]. In a 2020 study, Hu et al. proposed a pathophysiologic mechanism for the role of ABCA1 in regulating IOP, and an alternate function to its role in cholesterol homeostasis. They suggested that ABCA1 changes Cav1 expression, resulting in a decreased inhibitory influence of Cav1 on eNos, resulting in the increased nitric oxide production by eNOS and, ultimately, reduced IOP (
Table 2)
[19]. Additionally, they confirmed that ABCA1 had higher expression in the trabecular meshwork of POAG patients versus controls, confirming previous work suggesting the enhanced expression of ABCA1 as a diagnostic marker for glaucoma
[19][23]. Therefore, it is possible that variants in
ABCA1 and
NR1H3 result in altered IOP and aqueous outflow regulation.
The third most significant novel gene identified by Gao et al. in their 2018 GWAS was
MADD, a gene from the MAP kinase activating death domain
[16].
MADD plays a role in the tumor necrosis factor α (TNF-a) signaling pathway and, therefore, apoptotic signal transduction, by way of mitogen-activated protein kinase (MAPK)
[28].
MADD has been found to be upregulated in the retinas of POAG patients (
Figure 1)
[28][42]. This finding reflects those of increased TNF-a and TNF-a receptor 1 in glaucomatous optic nerve heads as measured by immunohistochemistry, suggesting a role for this signaling pathway in the neurodegenerative pathophysiology for POAG
[42][43][44].
Currently, there are no studies specifically linking
MADD expression and IOP. However, TNF-a and TNF-a receptor expression increased by nearly eightfold has been demonstrated in rats with elevated IOP compared to other animal models
[45]. Additionally, it has been suggested that TNF-a may indirectly mediate the cytotoxicity of elevated IOP via-microglial activation, and that stimulated glial cells may secrete TNF-a and nitric oxide to initially attenuate the effects of elevated IOP—despite the detrimental downstream effects of microglial activation such as the loss of aquaporin 4
[18][46][47][48]. Therefore, there may be an association between
MADD expression and IOP, as described by Gao et al.
[16]. Still, a 2010 analysis of TNF-a concentrations in the aqueous humor of glaucoma patients found a significant association between TNF-a concentrations and glaucoma patients (mean concentration 15.9 ± 3.6 pg/mL, range 1.7–57.6 pg/mL) but was unable to find a relationship between IOP and TNF-a concentration (
r = 0.163)
[49]. Therefore, the relationship between
MADD, and by extension TNF-a, and IOP remains unclear.
The final significant novel gene identified by Gao et al. was
SEPT9, a septin protein
[16]. Septins are a group of GTP-binding proteins that function in cell division and cytoskeleton formation; they have also been associated with neurodegenerative diseases
[50]. In 2013, Ghossoub et al. described the role of septins in retinal pigment epithelial cells, noting how SEPT2 forms a complex with SEPT7 and SEPT9, with the complex controlling ciliogenesis and, more specifically, the elongation of the primary cilium in cells of the retinal pigment epithelium (
Table 2)
[33].
The molecular mechanism linking septin activity and intraocular pressure has not been elucidated. It should be noted, though, that microtubule-associated protein 4 appears to play an antagonistic role with the activity of the SEPT2/SEPT7/SEPT9 complex in retinal pigment epithelial cells (
Figure 1)
[33]. Although not the same protein, one previous immunohistochemical study have shown that acutely increased IOP leads to a decrease in microtubule-associated protein 1 expression and a resulting decrease in the number of microtubules in optic nerve tissues, suggesting a possible mechanism by which cytoskeletal structures may be linked to IOP changes
[20].
While these four genes were identified as the most significant by Gao et al. in their 2018 GWAS, it is important to note that these genes are not conclusively the most important genetic markers of IOP. In fact, when Gao et al. calculated a heritability estimate for the UK Biobank sample limited to only significant genetic variants (
n = 671), the significant variants only explained 7.2% of variance
[16]. Additionally, when evaluated for pleiotropy, the 671 significant genetic variants also directly matched SNPs in the GWAS Catalog associated with PACG; age-related macular degeneration; other ocular characteristics such as central corneal thickness, axial length, and iris characteristics; and other systemic traits and conditions such as blood pressure, body mass index, type 2 diabetes mellitus, cancers, and immune disorders
[16].
Additionally, it is important to note, that IOP is not a perfect indicator for glaucoma, and therefore it cannot fully explain the heritability, nor the underlying molecular mechanisms, of all cases of POAG. In fact, a large subset of POAG patients do not have elevated IOP. These patients are referred to as having normal-tension glaucoma (NTG)—a unique classification of glaucoma where glaucomatous damage occurs independent of elevated IOP
[51]. In fact, Park et al. even suggested that the alteration of LMX1B transcription factor function may lead to glaucomatous damage independent of IOP alterations, as they noted that single-nucleotide polymorphisms (SNPs) of
LMX1B were also associated with NTG—and highlighting the danger of defining and evaluating POAG singularly through a lens of elevated IOP
[36].
3. Vertical Cup-to-Disc Ratio
In addition to IOP, CDR has been similarly suggested to be a “true” POAG endophenotype
[9]. VCDR is a reflection of the loss of retinal ganglion cells and axons, which results in the thinning of the neuroretinal rim and is represented clinically by alterations to the VCDR
[52]. Similar to IOP, VCDR is an imperfect indicator of disease. According to Tatham et al., VCDR has limited ability to detect glaucoma given the wide variability of VCDR in healthy eyes
[53]. Additionally, even though VCDR reflects retinal ganglion cell loss, VCDR is not a strong method for estimating quantitative measurements of retinal ganglion cell loss, largely due to wide variability of optic nerve head parameters
[53]. In fact, in a large population-based study of healthy eyes (
n = 6616), Hopley et al. found that the wide variability in normal CDR is influenced at least in part by the size of the optic disc
[54]. Still, according to a large systematic review from Hollands et al., increased CDR is a key risk factor for POAG, as the likelihood for POAG increases as CDR increases.
[55].
In their 2019 systematic review and meta-analysis, Asefa et al. calculated a heritability estimate for CDR at h
2 = 0.56 (0.44–0.68)
[8]. Importantly, they suggested that the wide variability in heritability estimates of CDR may be a reflection of the variability in cup size in the general population. Still, CDR shows relatively high heritability as a trait.
Genes significantly associated with CDR include, but are not limited to:
ABCA1,
ABG,
ADAMTS8,
ASAP1,
ASB7,
ATOH7,
ATOH7-PBLD,
BMP2,
CARD10,
CDC7-TGFBR3,
CDKN2B,
CDKN2B-CDKN2BAS,
CHEK2,
COL8A1,
CRISPLD1,
DCLK1,
DGKB,
DUSP1,
ELN,
ENO4,
EXOC2,
F5,
FAM101A,
GAS7,
HSF2,
PDZD2,
PLCE1,
PSCA,
RARB,
RERE,
RPAP3,
RPE65,
RREB1,
SALL1,
SCYL1,
SIX1,
SIX6,
SSSCA1,
TMTC2, and
VCAN [56][57][58][59][60][61][62]. Several of these genes, including
ABCA1,
ABG,
AFAP1,
CAV1,
GAS7, and
LMX1B have been independently associated with both IOP and CDR
[59][61]. Importantly, however, not all of these genes have also been independently associated with POAG.
In their 2017 GWAS and meta-analysis, Springelkamp et al. identified a variety of novel genes influencing CDR and other POAG endophenotypes. In order to better understand the molecular mechanisms behind these genes, they conducted a pathway analysis using DEPICT, a genetic association tool intended to predict gene function
[63]. Their findings were diverse; for example, they noted that genes associated with CDR, cup area and disc area were associated with biologic pathways associated with metabolic processes
[59]. Given redundancy between significant pathways, they clustered pathways into a series of meta-pathways, noting that gene sets centered on pathways involving cell differentiation and other developmental pathways
[59].
Interestingly, the meta-pathways Springelkamp et al. developed singled out pathways associated with abnormal fat cell and liver morphologies, noting that these pathways centered on
ABCA1 [59]. As described above,
ABCA1 is a transmembrane transporter with a role in cholesterol processing that has recently been linked to IOP regulation and aqueous humor dynamics (
Table 3)
[19]. So, while this recent development explains the linkage between
ABCA1, IOP, and the meta-pathways described by Springelkamp et al., the link to CDR is still unclear.
Table 3. Selection of genes associated with CDR and suggested molecular mechanisms.
| Gene |
GWAS Identified |
Suggested Mechanism |
Reference |
| ABCA1 |
Springelkamp et al., 2017 [59] |
Normal Function and Cell Death of Retinal Ganglion Cells |
Chen et al., 2014 [64] |
| ELN |
Han et al., 2021 [61] |
Alteration to Normal Function of Elastin, Leading to Optic Nerve Head Degeneration |
Gelman et al., 2010 [26] |
| ASAP1 |
Alipanahi et al., 2021 [65] |
Glial Cell-Mediated Retinal Ganglion Cell Loss |
García-Bermúdez et al., 2021 [66] |
| ATOH7 |
Nannini et al., 2018 [60] |
Alteration to Müller Cel Differentiation and Retinal Ganglion Cell Genesis |
Miesfeld et al., 2020 [25] |
In 2014, Chen et al. found that
ABCA1 was highly expressed in the retinal ganglion cell layer and is significantly higher in POAG patients. They suggested that this high expression indicates that
ABCA1 plays a role in the normal functioning and cell death of retinal ganglion cells
[64]. Given that CDR is a reflection of retinal ganglion cell death, it is likely that, in addition to its suggested role in regulating IOP and aqueous humor dynamics,
ABCA1 is linked to CDR through a molecular mechanism involving retinal ganglion cell death (
Figure 1). It is important to note that in 2018 Li et al. demonstrated that the liver X receptor/ABCA1 pathway may actually be protective against retinal ganglion cell apoptosis (
Table 2)
[22]. Their model, however, noted reduced ABCA1 retinal expression in response to acute IOP elevation, which may not be reflective of the role of ABCA1 protein in POAG and its link to CDR. Additionally, it is possible that associations between CDR and
ABCA1 may be reflective of IOP-mediated retinal ganglion cell death. Therefore, further research is surely warranted to better understand the true mechanistic relationship between these closely intertwined variables.
In 2021, Han et al. conducted the largest known GWAS for VCDR and vertical disc diameter (VDD) using by utilizing artificial intelligence (AI) in the form of a convolutional neural network model. Using this technology they were able to analyze VCDR and VDD from 282,100 images from the UK Biobank and another independent study and then were able to systematically use these studies to conduct a stronger GWAS of optic nerve head parameters
[61]. Using this data, they compared heritability estimates for VCDR as calculated from clinician gradings of CDR versus calculated from the AI-based GWAS. They found that the SNP-based heritability estimates were nearly 50% higher (VCDR h
2 = 0.22 vs. 0.35), indicating a future role for AI in heritability studies. In fact, they suggested that AI techniques may lead to higher heritability estimates in twin-studies
[61].
In addition to stronger heritability studies, the AI-assisted GWAS more than doubled the number of identified genetic loci for VCDR and VDD, similarly uncovering new mechanistic pathways. For example, Han et al. described new VCDR genes with possible links to retinal ganglion cell biology. Among these, a missense variant in the elastin gene,
ELN, was particularly noteworthy
[61].
Elastin is an extracellular matrix protein that has a significant role in tissue strength and elasticity
[67]. In the eye, elastin has been found extensively throughout the choroid, conjunctiva, meninges, muscle tendons and, most densely, in the sclera surrounding the optic nerve head (
Figure 1)
[26]. Given its function, elastin is a key component to normal ocular physiology as the sclera must be able to withstand the constant mechanical strain and stress induced by normal IOP and ocular blood flow pulsations (
Table 2)
[26]. Therefore, an alteration to the function of elastin and its ability to permit elastic structural movement and recovery, could have a dramatic degenerative effect. In the case of
ELN and VCDR, it is logical that alterations to the connective tissue of the lamina cribrosa could predispose a patient to glaucomatous damage and, as a result, increased VCDR.
Interestingly, in 2021, Alipanahi et al. ran a similar study using AI to enhance the power of a GWAS. Using a machine learning model based on 81,830 ophthalmologist-labeled fundus images to phenotype VCDR, they predicted VCDR on 65,680 participants from the UK Biobank. Then, using the machine learning-generated VCDR, they performed a GWAS to evaluate for genetic associations with VCDR. They uncovered 93 novel loci associated with VCDR
[65].
Among the novel genetic loci they uncovered was a SNP near
ASAP1, a locus previously associated with glioma
[65][68]. Recent research has demonstrated that glaucomatous retinal ganglion cell loss, and therefore clinical measurements of VCDR, may in some cases be mediated by glial cells
[66]. For example, the interplay of microglial activation and nitric oxide, as described previously, can be a beneficial response to elevations in IOP but may also lead to the downstream loss of aquaporin channels, leading to optic nerve degeneration
[46]. In the case of
ASAP1, Alipanahi et al. suggested that this association may indicate a mechanism by which glial cells mediate VCDR (
Table 2)
[65].
An important example of a glial cell-mediated mechanism that would lead to glaucomatous damage reflected clinically by VCDR is the gene
ATOH7, which has been significantly associated with VCDR
[56][57][69]. The ATHO7 protein, Atonal BHLH Transcription Factor 7, is a key transcription factor for the differentiation of Müller cells, the key retinal glial cell
[24][70]. According to Song et al., ATOH7 may promote the differentiation of retinal Müller cell-derived stem cells into retinal ganglion cells (
Table 2)
[24]. In fact, recent studies have shown that ATOH7 is a critical regulator of retinal ganglion cell genesis (
Figure 1). In 2020, Miesfeld et al. demonstrated that retinal ganglion cell genesis was strictly dependent on
ATOH7 expression (
Table 2)
[25]. Using a mouse model, they demonstrated how the number of adult retinal ganglion cells was directly correlated with
ATOH7 expression. Additionally, they noted how
ATOH7 mutants with decreased retinal ganglion cells experienced a variety of downstream effects and secondary malformations, including alterations to the retinal vasculature and optic nerve head parameters
[25]. These findings demonstrate that the association between
ATOH7 and VCDR may be mediated by the effect of the ATOH7 protein on glial cell differentiation into retinal ganglion cells, indicating one mechanism by which retinal glial cell alterations secondary to genetic alterations may predispose patients to alterations in VCDR.