3. ASAs with APs
3.1. Structure and Antimicrobial Activity for ASA with APs
Antimicrobial polymers (APs) have been designed to exhibit similar mechanisms of action as AMPs while diminishing AMPs’ disadvantages. APs are not degradable by enzymatic proteolysis as the AMPs, display controllable dose−dependent toxicity towards mammalian cells, have lower manufacturing costs than AMPs do, and can easily become available commercially from their facile production on a large scale following industrial synthetic protocols
[48][120][121][122][123][124][125]. Most APs, similar to AMPs, are positively charged in water solution; the electrostatic attraction drives the physical adsorption onto pathogenic microbes as the first step of their mechanism of action. Thereafter, they penetrate cell walls and membranes, leading to various degrees of antimicrobial activity and toxicity that can culminate in cell lysis with leakage of internal contents
[14][16][17][26]. The determination of APs specific cytotoxicity against mammalian cells has been a major requirement to establish their utility in vivo; it is important to evaluate whether APs used at a minimal bactericidal concentration (MBC) do not affect mammalian cells in culture
[10][126][127]. Certain APs showed dose−dependent cytotoxicity against human epithelial cells, lung fibroblasts, and monocytes
[128]. Nevertheless, their high antimicrobial activity at low doses has been the main motivation for the intense and extensive research on APs over the last twenty years
[10][13][14][15][17][129]; they have been often described as a promising platform for the development of next−generation antimicrobial agents
[120][122]. In addition, their flexible properties, facile synthesis, or modification from natural polymers, such as chitosan, gelatin, dextran, starch, or cellulose, also led to various alternative therapeutic applications
[20][130]. Among their important applications, APs have been used as adjuvants for vaccine design and antigen presentation
[126][127][129] and for gene and drug delivery
[131]; interestingly, aminated microcrystalline cellulose killed melanoma and breast cancer cell lines in culture
[131]. Excellent overviews on the synthesis and preparation of cationic polymers are available
[48][130][132][133].
Most cationic polymers bear amine functions that can be protonated, such as polyethyleneimine (PEI), poly−L−(lysine) (PLL), chitosan, and poly [2−(N,N−dimethylamino)ethyl methacrylate] (PDMAEMA)
[134]. While these polymers have inherent cationic charges, others have been developed by introducing cationic moieties such as aminated cellulose, which, compared to chitosan, represent a novel cationic cellulose derivative with improved mucoadhesive properties as well as sufficient hydration at physiological pH
[135]. Chitosan derivatives bearing quaternary ammonium moieties and displaying good antimicrobial activity have also been developed
[136].
Antimicrobial cationic polymers mainly contain two functional components: the cationic and the hydrophobic groups. The antimicrobial activity is influenced by the type, amount, location, and distribution of these two components; the structure–function relationship for AP could provide some guidelines for developing molecular engineering of antimicrobial cationic polymers with tailor−made structures and functions
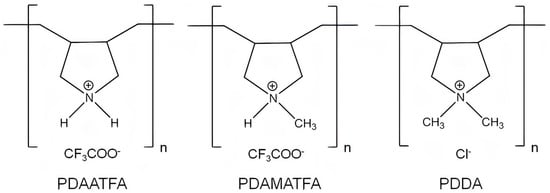
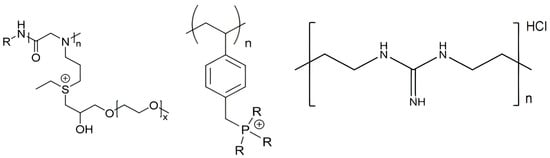
[137]. For example, the chemical structures of poly (diallyldimethylammonium chloride) (PDDA) derivatives with different hydrophobic–hydrophilic balances such as poly (diallylammonium trifluoroacetate) (PDAATFA), poly (diallylmethylammonium trifluoroacetate) (PDAMATFA), and PDDA itself are shown in
Figure 7 [17]; their hydrophobic–hydrophilic balance and their antimicrobial activity against Gram−negative bacteria increase from left to right
[17][138].
Figure 7. Chemical structures of some PDDA derivatives with increasing hydrophobic–hydrophilic balance from left to right. Polymers are poly (diallylammonium trifluoroacetate) (PDAATFA), poly (diallylmethylammonium trifluoroacetate) (PDAMATFA) and poly (diallyldimethylammonium chloride) PDDA. Reproduced from
[17].
The activities of the PDDA derivatives on
Figure 7 against Gram−positive bacteria or fungus were not so clearly dependent on the hydrophobic–hydrophilic balance of the molecule, possibly due to superimposed effects of AP molecular weight and/or the nature of the molecular composition and the nature of the microbes cell wall for different species; against fungus, no effect of the molecular weight or the hydrophobic–hydrophilic balance were apparent; the fungus was very sensitive to all PDDA derivatives
[16][17][138].
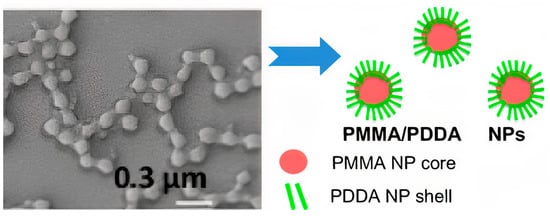
PDDA immobilization in biocompatible poly (methyl methacrylate) (PMMA) NPs diminished its antimicrobial activity to a certain extent; PMMA/PDDA NPs synthesis from emulsion polymerization of the methyl methacrylate (MMA) monomer in the presence of PDDA yielded interesting core−shell NPs; the free PDDA molecules showed lower minimal microbicidal concentrations (MMC) than the immobilized ones
[17]. The core−shell nature of PMMA/PDDA NPs was an interesting finding; the hydrophobic, neutral PMMA polymeric core became surrounded by a shell of the hydrophilic, cationic polyelectrolyte PDDA, as shown in
Figure 8. Apparently, the cationic NPs were not as efficient as free PDDA to penetrate the microbes’ cell walls and membranes
[17][23].
Figure 8. Scanning electron micrograph of poly (methyl methacrylate) PMMA/poly (diallyl dimethyl ammonium) chloride PDDA antimicrobial nanoparticles (PMMA/PDDA NPs) and schematic representation of their core−shell structure at low ionic strength. Reproduced from
[17]. The NPs were obtained from emulsion polymerization of methyl methacrylate (MMA) in the presence of PDDA.
Besides the quaternary ammonium, cationic moieties in antimicrobial polymers could be sulfonium
[139][140], guanidinium
[125][128][141], or phosphonium
[142][143]. Polymeric sulfonium salts exhibited high antibacterial activity against Gram−positive bacteria but were less active against Gram−negative bacteria
[139]. It was found that the activity of the polymeric sulfonium salts was much higher than that of the corresponding monomers, particularly against S. aureus.
Figure 9 shows some chemical structures for cationic APs with sulfonium, phosphonium, or guanidinium as the cationic moiety.
Figure 9. From left to right, some cationic polymers with different cationic moieties such as sulfonium
[139][140], phosphonium
[142], and guanidinium
[141][144].
The sulfonium polypeptoid on the left (
Figure 9) was obtained via ring−opening polymerization and post−modification strategy with excellent biological performance for the treatment of the infections caused by S. aureus; it contained both sulfonium and oligo (ethylene glycol) (OEG) motifs and displayed high selectivity for the pathogens over mammalian red blood cells
[140]. Similar to natural AMPs that contain cationic and amphipathic moieties, several synthetic antimicrobial polymers named polypeptoids have been proposed. They were polymers analogous to AMPs with the advantages of facile synthesis at low cost and excellent stability against degradation in vivo. These peptidomimetic polymers had, for example, an N−substituted glycine backbone similar to the polypeptoid shown on the left in
Figure 9 [140].
Various cationic polymers with quaternary ammonium or phosphonium, which possessed high antimicrobial activities in solution, exhibited a significant decrease in their antimicrobial efficiency after crosslinking or solubilization loss
[17][145]. The antimicrobial activity of water−insoluble polycations could be preserved as long as the polymeric chains were long and flexible for penetration through the bacterial membranes. In a series of water−insoluble N−alkyl−N,N−dimethyl de−oxy ammonium celluloses, those modified by N,N−dimethyl dodecyl ammonium exhibited antimicrobial activity, while those modified by N,N−dimethyl butyl ammonium did not
[146]. PDDA immobilization as the outer shell of PMMA nanoparticles core also reduced antimicrobial activity in comparison to the activity of free PDDA in solution
[17]. Another interesting example of phosphonium−modified polymer were some inulin derivatives; inulin is a natural, renewable, biodegradable, and water−soluble carbohydrate recently modified with quaternary phosphonium salt to impart antifungal activity to the molecule. The antifungal activity increased with the alkyl chain length of the grafted quaternary phosphonium salt
[147].
3.2. Biomedical Applications for ASA with APs
Synergistic antimicrobial activity against ESKAPE pathogens was reported for combinations of quaternary ammonium and guanidinium homopolymers
[148]. Guanidinium polymers were successfully used to target intracellular, multidrug−resistant Staphylococcus aureus
[149]. Non−leaching polyacrylate and guanidine−based copolymer NPs with 80–130 nm mean diameter were synthesized by emulsion polymerization with acrylate and glycidyl−methacrylate monomers and modified by oligoguanidine; NPs and their films presented long−term antimicrobial activity
[150]. The antimicrobial copolymer of polyhexamethylene guanidine hydrochloride and polypropylene glycol diglycidyl ether adhered onto cotton fabrics both by physical adsorption and covalent binding, resulting in durable antimicrobial properties against Escherichia coli and Staphylococcus aureus; antimicrobial activity remained unchanged even after laundering the fabrics with detergent solution
[151]. New chitosan derivatives bearing guanidinium functions were synthesized following different synthesis strategies. N−guanidinium chitosan acetate and N−guanidinium chitosan chloride were synthesized by direct reaction between chitosan and cyanamide in the presence of scandium (III) triflate. The synthesis of N−guanidinium chitosan (N,N′−dicyclohexyl) chloride and N−guanidinium chitosan (N−(3−dimethylaminopropyl)−N’−ethyl hydrochloride) chloride involved the reaction of chitosan with carbodiimides in ionic liquid. All newly guanylated chitosan derivatives displayed high antimicrobial activity in comparison with neat chitosan
[152]. Guanidine−based polymers imparting antimicrobial activity to polysaccharides, such as cellulose, starch, and cyclodextrin, have been recently overviewed
[153].
The accepted mechanism of action for cationic polymers involves the same membrane disruptive effects observed for AMPs; major events are adsorption into the bacterial cell surface, penetration into the cell wall, and insertion into the cytoplasmic membrane (due to hydrophobic group of the polymer) with membrane disruption, leakage of cytoplasmic contents, and eventually, cell lysis
[14][96][121][154][155][156]. Mechanisms of action for APs and AMPs indeed decreased the odds of creating resistant bacteria
[7][9][51][52][59].
Antimicrobial biopolymers were an important branch in this field; their exclusive qualities usually include being natural, biodegradable, biocompatible, cheap and extracted from biomass−derived waste, and, some of them, being both antibacterial and antifungal agents
[8][157][158]. The interest in these molecules has been growing along with environmental concerns
[159]. Some examples of biopolymers are: cellulose, the most abundant one in nature; chitosan, a versatile polymer attainable by treating chitin from the crustacean shell waste generated by the seafood industry; and lignin, a byproduct of the paper industry with great qualities, including antioxidant activity and high thermal stability
[158][159][160][161]. Recent studies with hydroxypropyl methylcellulose (HPMC)/lignin and HPMC/lignin/chitosan films had positive results, with antimicrobial effect against both Gram−positive and Gram−negative at 35 and 0–7 °C
[158]. Even though most biopolymers came with interesting advantages, some of them have their problems, such as chitosan sensitiveness to temperature and pH
[162]. Biopolymers could eventually replace synthetic ones without critical side effects
[159].
The biomedical applications for antimicrobial polymers required thromboresistant materials also able to avoid the formation of biofilms
[163]. In biomedical devices such as catheters, intravascular grafts, extracorporeal circuits and membrane oxygenators, the adsorption of serum proteins onto these blood−contacting materials may trigger the blood coagulation cascade, whereas the contact with infective pathogens may cause the formation of biofilms and infection.
A few examples in the literature deal with the production of materials displaying both thromboresistant and anti−biofilm properties. The sulfonated polymers and sulfated glycosaminoglycan have been widely recognized as heparin−mimetic components since they show similar functionalities as heparin, displaying anticlotting and antithrombotic activities, the stabilization of growth factors, and the promotion of angiogenesis
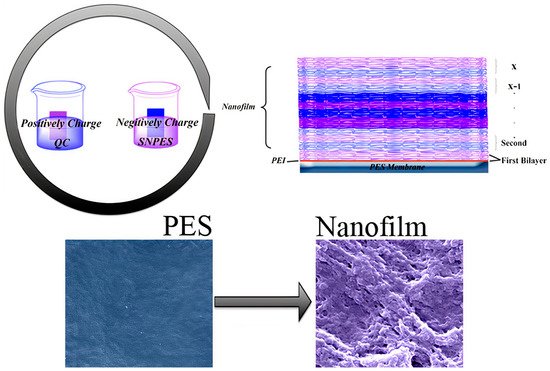
[164][165]. Some combined polymers joining multiple functional groups on one surface, such as a synthetic heparin−mimetic polymer or hydrophilic polymer brushes (e.g., PEG) with antibacterial quaternary compounds (QAC), were described. The layer−by−layer assembly of sulfonic amino poly (ether sulfone) (SNPES) and quaternized chitosan (QC) yielded multilayers. Additionally, when submitted to systematic tests for antithrombotic and antimicrobial activity, the multilayers showed that the heparin−mimetic multilayer−coated membrane suppressed adsorption of bovine serum fibrinogen, platelet adhesion, and activation, prolonged clotting times, and reduced activation of blood complement. Furthermore, the antibacterial test suggested that the multilayer−coated substrates exhibited activity against Escherichia coli and Staphylococcus aureus
[166].
Figure 10 illustrates the preparation of these multilayered coatings.
Figure 10. Scheme of layer−by−layer assembly of sulfonic amino poly (ether sulfone) (SNPES), a heparin−mimetic polymer, and quaternized chitosan (QC), an antimicrobial polymer, onto poly (ether sulfone) (PES) membrane substrates. SNPES and QC are negatively and positively charged, respectively. Reproduced with permission from
[166]. Copyright 2015 American Chemical Society.
The layer−by−layer approach has also been useful to impart anti−biofilm property to materials; deposition of PDDA and poly (acrylic acid) (PAA) layers with an outer PAA layer onto polyester fabric prevented S. aureus adhesion to the fabric and allowed removal of bacteria by water rinse
[167].
Recent progress in the biomedical applications of polydopamine (PDA) nanostructures, such as drug delivery, photothermal therapy, bone and tissue engineering, cell adhesion, and antimicrobial uses, were recently reviewed
[168]. Rough PDA films on various substrates such as reverse osmosis filtration membranes
[169], glass, plastic, stainless steel, and gauze showed remarkable antibacterial activity as compared with smooth PDA films
[170]. The antifouling and antibacterial properties were attributed to the fact that the PDA film with positively charged amine groups would be responsible for the interaction with bacterial cell walls at high pH, causing cell rupture. Moreover, the rough surface of PDA exhibited higher particle contact with substrates during vigorous shaking and thus exhibited more bactericidal action
[170]. Coatings cast onto silicon wafers from PMMA/PDDA nanoparticles also displayed a correlation between contact points between cells and films and the antimicrobial activity
[31].
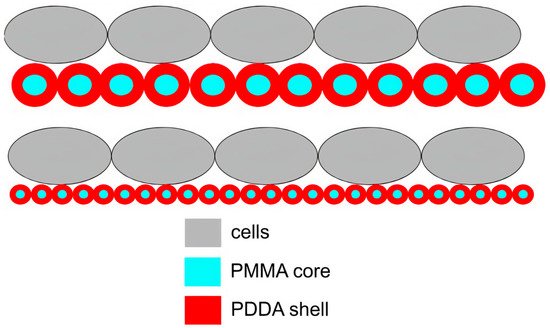
Figure 11 illustrates the compared frequency of contacts between cells and coatings from two coatings cast onto silicon wafers from PMMA/PDDA dispersions as reproduced from reference
[31].
Figure 11. Scheme for the interaction between cells and coatings cast from core−shell nanoparticles. Nanoparticles in the coatings optimized antimicrobial activity due to a higher frequency of multipoint interactions between poly (diallyl dimethyl ammonium) chloride (PDDA) shell (in red) and cells (in grey) than the one for the larger particles. Reproduced from
[31].
Another interesting approach recently reviewed was covalently binding or combining by physical adsorption AMPs and functional antimicrobial polymers
[171], e.g., chitosan
[172] or polydopamine
[173]. The conjugation of AMPs into functional polymers broadened the spectrum of antimicrobial activity, including activity against MDR bacteria, reduced toxicity, and offered more functionalities for developing multifunctional biomedical hydrogels, polymer vesicles, or polymer micelles
[171]. For example, the peptide anoplin, extracted from wasp venom, was covalently bound to chitosan to create a highly antimicrobial yet selective and nonhemolytic agent
[172]. The conjugate displayed greater antibacterial activity when compared to anoplin only, especially against Gram–negative bacteria
[172]. Another example of the peptide–polymer conjugate was a thin layer of PDA deposited onto a surface of polydimethylsiloxane (PDMS) to ease the attachment of peptide CWR11, creating a PDMS/PDA/CWR11 slide; CWR11 was attached to PDA through nucleophilic addition via thiol or amine group at either end of the peptide chain or through physical adsorption onto the PDA surface; the attachment of CWR11 conferred the PDA–coated PDMS surfaces a high antimicrobial activity against E. coli, S. aureus, and P. aeruginosa; the antifouling property was also present as determined by seeding fluorescently labeled–P. aeruginosa onto the slides of PMDS/PDA/CWR11; the material might be applied in catheters to prevent catheter–associated urinary tract infections caused by the development of biofilms on its surface
[173].
The conducting and hydrophilic polymer polyaniline (PANI) has been considered promising for applications in biomedicine because of its high electrical conductivity and biocompatibility. However, PANI’s low processability and degradability led to its combination with various biopolymers and nanomaterials as blends and nanocomposites, respectively. Biomedical applications of conductive PANI−based nanocomposites were available in antimicrobial therapy, drug delivery, biosensors design, nerve regeneration, and tissue engineering
[174][175]. PANI materials have been used in photothermal therapy (PDT) for treating tumors or infections; the incidence of near−infrared radiation (NIR) onto PANI materials led to photothermal ablation of cancer cells or bacteria death
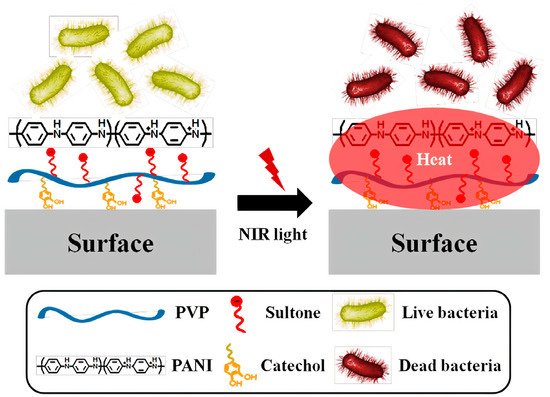
[176]. For example, catechol−conjugated poly (vinyl pyrrolidone) sulfobetaine (PVPS) and PANI tightly linked by ionic interaction (PVPS:PANI) has been proposed as a novel photothermal antibacterial agent for surface coating, able to absorb broadband NIR light; the coating released eminent photothermal heat for the rapid killing of surface bacteria
[177].
Figure 12 illustrates the photothermal effect of PVPS:PANI coatings on bacteria
[177].
Figure 12. Scheme for the preparation and application of poly (vinyl pyrrolidone) sulfobetaine:poly aniline (PVPS:PANI) coating and near−infrared radiation (NIR) for the photothermolysis of bacteria. Reprinted with permission from
[177]. Copyright 2015 American Chemical Society.
Similarly to PVPS: PANI coatings on
Figure 12, the photothermal antimicrobial effect was also used to kill bacteria adhered to fabrics of polyethylene (PE) impregnated with poly ethylene imine−poly pyrrole NPs able to absorb the near−infrared light, thereby heating the fabric and killing adsorbed bacteria. Moreover, the fabric became washable, reusable, breathable, biocompatible, and photothermally conversable for active eradication of pathogenic bacteria
[178].
A three−dimensional liver scaffold was fabricated from a chitosan/gelatin (CG) solution cross−linked with glutaraldehyde and showed a porous structure similar to the extracellular matrix that facilitated hepatocyte adhesion and proliferation; this CG scaffold had high hepatocyte biocompatibility and mechanical strength but also maintained hepatic functions and viability in in vitro cultures; especially, this liver scaffold revealed high potential for further bioartificial liver design in the near future
[179].
Skin traumas such as burns and wounds are susceptible to microorganisms invasion; recent studies succeeded in treating E. coli and S. aureus infected skin with antimicrobial polymers in hydrogels, coatings, nanofibers and nanogels formulations
[180][181][182][183][184]. The development of effective wound dressings was essential for speeding up wound healing.
Rectorite, a type of layered silicate, yielded interlayered nanocomposites with positively charged polymers such as quaternized chitin; these composites combined with cellulose fibers created functional sponges with antibacterial and hemostatic properties for wound−healing applications; the in vivo animal tests demonstrated that the sponges rapidly induced hemostasis in a rat tail amputation test, making them superior to the traditional hemostatic materials; in addition, the sponges could substantially promote collagen synthesis and neovascularization, thereby accelerating wound healing 3 days earlier than gauze. This multi−functional biomedical material, fabricated using natural substances, showed great potential to be used for wound healing
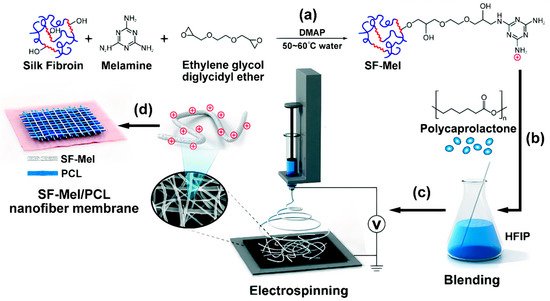
[185]. Another interesting antimicrobial polymer, melamine−modified silk fibroin (SF–Mel), has been used to produce films with poly caprolactone (PCL) nanofibers via the electrospinning technique. These films were hemocompatible and noncytotoxic, exhibiting broad−spectrum antibacterial activities against both Gram−negative (Escherichia coli) and Gram−positive bacteria (Staphylococcus aureus). In vivo evaluations showed accelerated wound healing by promoting re−epithelialization, collagen deposition, and vascular reconstruction; chemically grafting melamine on the side chains of silk fibroin could improve the antimicrobial properties due to the existence of positively charged amine groups derived from melamine
[180].
Figure 13 illustrates the preparation of the PCL/SF–Mel wound dressings
[180].
Figure 13. The film with cationic polymer useful as an antimicrobial wound dressing. (
a) Melamine−modified silk fibroin (SF–Mel) was synthesized by covalent conjugation between silk fibroin and melamine. (
b) Polycaprolactone (PCL) blended with SF–Mel enhanced the mechanical properties. (
c) SF–Mel/PCL nanofiber films were fabricated via electrospinning. (
d) Nanofiber membrane comprising SF–Mel/PCL was constructed as a wound dressing for skin repair. Adapted with permission from
[180]. Copyright 2019 The Royal Society of Chemistry.
Cryopolimerization of dopamine in the presence of quaternized chitosan (QC) yielded QC/ polydopamine (PDA) cryogel, with PDA concentrations varying from 0.5 to 4.0 mg/mL so that the highest PDA concentrations yielded the best antibacterial and antioxidant activities plus near−infrared photothermal effect; moreover, these cryogels exhibited much better hemostasis than gauze and gelatin sponge in vivo in three different models: a rat liver injury model, a rabbit liver section model, and a pig skin laceration model; there was improved blood cell and platelet adhesion, with quick nonpressing surface hemostasis and wound healing
[181]. Tributylammonium alginate (TBAH−Alg) salt was deposited onto modified cationic polyurethane surfaces (CPU) through supramolecular ionic interactions to create a wound dressing. Both CPU and CPU/TBAH−Alg showed large inhibition zones against bacteria in agar diffusion; in vivo experiments in wound models treated with the CPU/TBAH−Alg dressings reduced the persistent inflammatory phase and improved re−epithelialization, collagen deposition, and mature blood vessel formation, showing better results than commercial dressing Tegaderm
[182].
3.3. Other Applications for ASA with APs
ASAs with antimicrobial polymers were also involved in the food packaging, fabrics and textile industries, in addition to water treatment
[96][157].
In water treatment, the use of organic polyelectrolytes included a myriad of examples of the benefits of polymer use in conventional sedimentation and filtration; however, the influence of polymer chemical structure on performance has been investigated superficially
[186]. Organic coagulants and flocculants were usually water−soluble polymers (polyelectrolytes) originated from various natural macromolecular compounds, including polyamines, PDDA, dimethylamine, and polyacrylamides
[187]. Among the flocculants, only a few exhibited both antimicrobial and coagulation properties; PDDA was among those able to impart both properties to materials used for water treatment
[8][13][14][17][188].
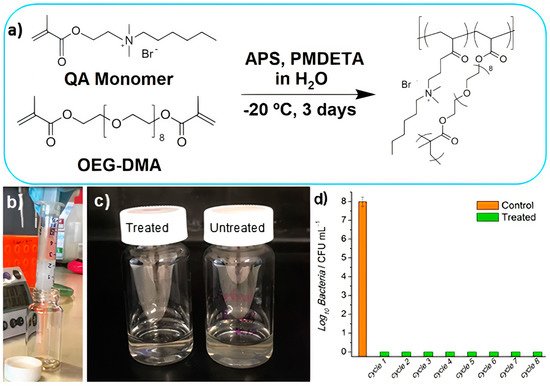
Macroporous antimicrobial polymeric gel (MAPG) containing quaternary ammonium in its chemical structure was synthetized through cryopolymerization; a hydrogel (HG) with the same chemical composition was also prepared for comparison. Firstly, a quaternary ammonium (QA) methacrylate monomer bearing a hydrophobic n−hexyl tail was synthesized
[189]. This was an adequate antimicrobial combination of cationic and hydrophobic groups in the monomer to synthesize the polymer: the n−hexyl group was selected as sufficiently hydrophobic to cause membrane disruption, while the cationic moiety implemented adsorption to the bacteria cell wall
[189]. The polymerization is shown in
Figure 14. First, the QA monomer was synthesized via quaternization reaction between 2−(dimethyl amino) ethyl methacrylate and 1−bromohexane; second, the polymerization of the QA monomer via free−radical polymerization in the presence of a redox radical initiator (i.e., ammonium persulfate), coinitiator N,N,N′,N′′,N′′−pentamethyldiethylene triamine (PMDETA), and a cross−linkable monomer (i.e., oligoethylene glycol dimethacrylate (OEG−DMA)) in water was carried out at subzero temperature (
Figure 14a). Filtration of contaminated water using MAPG produced pure water without bacteria (
Figure 14b–d)
[189]. Cryogelation required only simple mixing of chemicals, hence making the whole process potentially viable for industrial−scale preparation.
Figure 14. Macroporous antimicrobial polymeric gel (MAPG) for water treatment. (
a) MAPG synthesis via free radical polymerization at subzero temperature. (
b) Image of macroporous antimicrobial polymeric gel (MAPG) in a syringe and E. coli−contaminated water passing through it. (
c) Image of treated water after passing through the syringe. Compared to the untreated, cloudy water due to the presence of bacteria, the treated water was clear. (
d) The syringe was subjected to 8 continuous cycles of percolation with E. coli−contaminated water; the recovered water was analyzed via colony−forming unit (CFU) counting. No viable bacteria were detected in the water that passed through the syringe. APS is ammonium persulfate, QA monomer is the quaternary ammonium ethyl methacrylate monomer, PMDETA is the coinitiator N,N,N′,N′′,N′′−pentamethyldiethylenetriamine, and OEG−DMA is oligoethylene glycol dimethacrylate. Adapted from
[189].
Polymers, which act as the primary substrate for face masks, could be fine−tuned to impart bio−active and bio−passive properties to the fabrics. The active moieties such as N−halamines, QACs, PEI, benzophenone (BP), polypyrrole, and inorganic groups, such as metals, have been incorporated to yield various antimicrobial polymers suitable for making a reusable facemask
[190][191]. Among these, N−halamine and QACs have proven and powerful activity against a broad spectrum of microorganisms. Bath coating, spray coating, and immobilization via carriers have been employed to yield QACs modified antimicrobial fabrics. Direct polymerization of monomers and covalent attachment of QACs was expected to enhance the stability of the coating and the performance. N−halamines had high effectiveness in short contact times in antimicrobial fabrics
[192]. However, the real field applicability of N−halamine on face masks has not been explored yet. Natural compounds and antimicrobial peptides are promising molecules due to less ecotoxicity and proven antimicrobial properties. Recently, the inactivation by oxygen singlet of severe acute respiratory syndrome coronavirus 2 (SARS−CoV−2) using light on synthetic conjugated polymers and oligomers was reported; five representative conjugated oligomers and polymers from an array of phenylene ethynylene−based cationic and anionic conjugated materials against SARS−CoV−2 revealed that light activation of the materials at the wavelengths where they absorb gave rise to moderate to very strong inactivation of the virus. Furthermore, no dark inactivation of the virus for three of the five materials/compounds occurred for the quaternary ammonium derivatives. Therefore, the generation of reactive oxygen species definitely inactivated the virus; the incorporation of these materials in wipes, sprays, masks, and clothing and other personal protection equipment would possibly be useful in preventing infections and the spreading of the virus and future outbreaks from similar viruses
[193]. A more recent report on the development of a non−woven face mask filter fabricated with a coating of benzalkonium chloride, a quaternary ammonium compound, was able to inactivate more than 99% of SARS−CoV−2 particles in one minute of contact and also methicillin−resistant Staphylococcus aureus and Staphylococcus epidermidis; this would solve the pressing problem of commercial face masks that contained filters not capable of inactivating either SARS−CoV−2 or multidrug−resistant bacteria
[194].
4. Conclusions
Antimicrobial polymers, such as APs and AMPs, have been widely explored as materials for biomedical applications. Their chemical structure usually contains both cationic and hydrophobic moieties, exhibiting unlimited potential to fight microbial resistance against available antibiotics. In terms of their potential shortcomings, in vivo AMPs necessitate protection from proteolytic enzymes and rapid degradation, whereas APs still require improvements in terms of their biocompatibility. The similar mechanisms of action found in APs and AMPs involve adsorption to the cell wall, penetration across the cell membrane, and microbe lysis. The synthetic procedures, chemical stability, and improved adsorption of APs—the latter due to their multipoint attachment to microbes—represent significant advantages in comparison to the expensive synthetic pathways for procedure scaling, poor yield, and subpar in vivo stability of AMPs. ASAs with APs and AMPs have also been found useful in water treatment and the production of fabrics and textiles endowed with suitable antimicrobial properties, e.g., face masks and air filters, which have become important and oftentimes crucial defenses in an era of pandemics.