1000/1000
Hot
Most Recent

In the relatively short history of anti-tumor treatment, numerous medications have been developed against a variety of targets. Intriguingly, although many anti-tumor strategies have failed in their clinical trials, metformin, an anti-diabetic medication, demonstrated anti-tumor effects in observational studies and even showed its synergistic potential with immune checkpoint inhibitors (ICIs) in subsequent clinical studies. Looking back from bedside-to-bench, it may not be surprising that the anti-tumor effect of metformin derives largely from its ability to rewire aberrant metabolic pathways within the tumor microenvironment. As one of the most promising breakthroughs in oncology, ICIs were also found to exert their immune-stimulatory effects at least partly via rewiring metabolic pathways. These findings underscore the importance of correcting metabolic pathways to achieve sufficient anti-tumor immunity. Herein, we start by introducing the tumor microenvironment, and then we review the implications of metabolic syndrome and treatments for targeting metabolic pathways in anti-tumor therapies. We further summarize the close associations of certain aberrant metabolic pathways with impaired anti-tumor immunity and introduce the therapeutic effects of targeting these routes. Lastly, we go through the metabolic effects of ICIs and conclude an overall direction to manipulate metabolic pathways in favor of anti-tumor responses.
Of all diseases, cancer has long been one of the major health concerns in spite of extensive research exploring ways to tackle it. Due to its abnormal growth and deadly metastasis irrespective of limited oxygen and nutrients, the deregulating cellular energetics and the immunoevasion nature of cancer have been identified, which are largely derived from its ability to recruit normal cells that constitutively create the “tumor microenvironment” (TME) [1]. The TME is characterized by a complex interplay between tumor cells and their surrounding neighbors, including stromal cells, extracellular matrix, adipocytes, mesenchymal stem cells, blood vessels, macrophages, T cells, B cells, cytokines, exosomes and metabolites. All the components within the TME contribute to building a 3-dimensional structure with gradients of oxygen tension and availability to nutrients (as shown in Figure 1) [2][3], which favors the development of tumors in multiple aspects such as local progression and distal metastasis [4][5][6][7][8][9]. Importantly, although immune cells are known to protect us from tumors through their immunosurveillance and tumoricidal nature [10][11], multiple factors within the TME may not only hinder their antitumor function but also skew them to construct an immunosuppressive environment in favor of tumor growth.
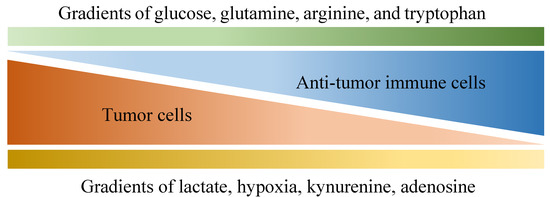
Figure 1. Various gradients within the tumor microenvironment that are differentially associated with anti-tumor activity and tumor growth. Tumor cells are known for their aberrant metabolic activity that leads to local depletion of a variety of nutrients, including glucose, glutamine, arginine and tryptophan, which effectively hinder anti-tumor activities provided by immune cells that also depend on these nutrients. In addition, metabolites such as lactate, kynurenine and adenosine are released by tumor cells, dampening anti-tumor immunity along with hypoxia.
In the devastating battlefield against tumors, a variety of protumoral factors and immunosuppressive mechanisms have been identified, of which the immunosuppressive cells, exosomes and the co-inhibitory signals play central roles to allow tumor progression. To begin with, the immunomodulatory cells, including but not limited to regulatory T (Treg) cells and M2 macrophages, accumulate in the TME and diminish T cell anti-tumor immune responses [12][13]. Treg cells are famous for their immunosuppressive effects on not only aberrant immune responses against self-antigens but also anti-tumor immune responses, in both laboratory and clinical studies [14][15][16]. Treg cells are believed to modulate immune responses through expressing immunosuppressive cytokines (including transforming growth factor (TGF)-β, interleukin (IL)-10, IL-35), immune checkpoints (such as cytotoxic T-lymphocyte-associated antigen (CTLA)-4; programmed death (PD)-1) and other co-inhibitory receptors, as extensively reviewed by previous studies [17][18]. Furthermore, Treg cells are also capable of inducing tolerogenic dendritic cells (DCs) that are linked to T cell exhaustion, and can release cytotoxic agents such as perforin and granzyme [19][20][21][22][23]. Therefore, the aberrant accumulation of Treg cells in TME impairs anti-tumor immunity through various mechanisms [24][25], facilitating tumor growth and progression. Besides Treg cells, the unwanted immunosuppression within the TME can also be affected by tumor-associated macrophages (TAMs) that are majorly driven by cytokines such as IL-4 or IL-13, which have also earned their “M2-like” naming [26][27][28]. Orchestrated by signals from tumor cells, T cells as well as stroma [29][30], TAMs play a protumoral role in the TME by promoting tumor metastasis through promoting angiogenesis as well as extracellular matrix remodeling [31][32]. Moreover, TAMs also exert their profound immunosuppressive effects through expressing a variety of inhibitory ligands and cytokines, including PD-L1, PD-L2, B7-1, B7-2, HLA-G, HLA-E, IL-10 as well as TGF-β, as clearly reviewed by previous studies [33][34]. Besides immunosuppressive cells, previous studies further reveal the vital roles of exosomes and co-inhibitory molecules to promote tumor growth and metastasis in many different ways.
The aberrant accumulation of immunosuppressive cells within the TME is thought to be affected by both exosomes and co-inhibitory signals derived from the tumor cells. Exosomes are extracellular vesicles with bi-layered membrane that range between 30-100 nm in diameter [35], while patients with cancer, especially of the ones with poor prognosis, are often found with higher numbers of them [36]. Tumor-derived exosomes have been detected in many different types of cancers, and they can not only impair anti-tumor immunity within the TME but also educate bone marrow-derived progenitor cells to facilitate distant metastasis [37][38][39][40]. On the other hand, the expression of co-inhibitory molecules such as PD-L1 have been observed on various kinds of cancer cells [41], which correlates with poor clinical outcomes of many patients with cancer [42][43]. Importantly, since the co-inhibitory checkpoint molecules expressed by both tumors and immunosuppressive cells within the TME dampens anti-tumor immunity of T cells [44][45][46][47], immune checkpoint inhibitors (ICIs) such as the antibodies directed against CTLA-4, PD-1 and PD-L1, are developed into encouraging treatments against various tumors, most notably melanoma and non-small cell lung cancer [48][49][50][51]. For instance, a pooled meta-analysis assessing long-term survival of 1861 advanced melanoma patients estimated a 3-year survival rate of 22% for patients receiving anti-CTLA4 ipilimumab [52], which evidently outperformed other chemotherapy such as dacarbazine, where the 3-year survival rates were only around 12% [53]. However, despite the game-changing efficacies of ICIs against tumors in clinical scenarios, they still face improvable downsides, including the suboptimal long-term response rates due to both innate and acquired resistance [54], as well as the lack of a reliable predictive biomarker [55]. Therefore, a variety of combinatory therapeutic strategies, along with the discovery of potential biomarkers, have been tested out to overcome the limitations, and targeting the metabolic pathways within the TME has appeared as one of the emerging candidates that have synergistic potential.