1000/1000
Hot
Most Recent

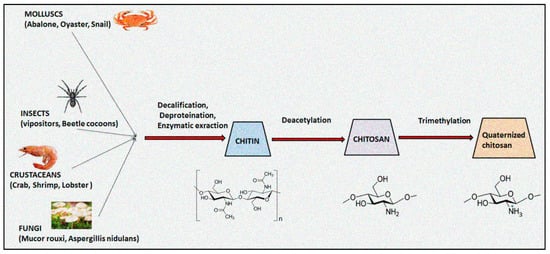
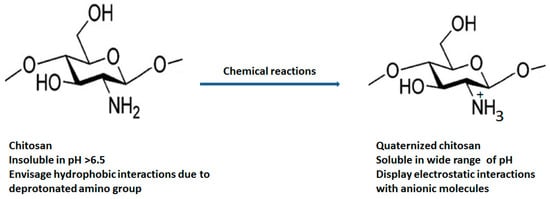
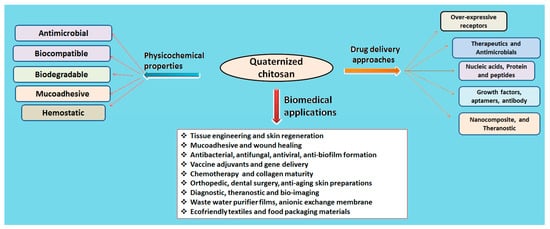
The natural polymer chitosan is the second most abundant biopolymer on earth after chitin and has been extensively explored for preparation of versatile drug delivery systems. The presence of two distinct reactive functional groups (an amino group at C2, and a primary and secondary hydroxyl group at C3 and C6) of chitosan are involved in the transformation of expedient derivatives such as acylated, alkylated, carboxylated, quaternized and esterified chitosan. Amongst these, quaternized chitosan is preferred in pharmaceutical industries owing to its prominent features including superior water solubility, augmented antimicrobial actions, modified wound healing, pH-sensitive targeting, biocompatibility, and biodegradability. It has been explored in a large realm of pharmaceuticals, cosmeceuticals, and the biomedical arena. Immense classy drug delivery systems containing quaternized chitosan have been intended for tissue engineering, wound healing, gene, and vaccine delivery.
|
Parameters |
Responses |
|---|---|
|
Degree of quaternization (<65%) |
Increased cytotoxicity |
|
Increased mucoadhesiveness |
|
|
Decreased anticoagulation effect |
|
|
Degree of quaternization (≥20%) |
Increased antimicrobial action in pH = 7.2 |
|
No effect on antimicrobial action in acidic pH |
|
|
Degree of substitution (<1%) |
Increased antioxidant property |
|
Degree of substitution (<25%) |
Increased antithrombin action and acid-binding capacity |
|
Degree of substitution (>1%) |
Decreased moisture absorption and retention ability |
|
High concentration |
Increased particle size, aggregation, zeta potential, cytotoxicity |
|
Decreased knockdown efficiency and poor transfection efficacy |
|
Components |
Purpose |
Research Outcomes |
References |
|---|---|---|---|
|
HTCC and PVA |
Retention of non-enveloped virus on the highly charged HTCC/PVA nanofibers. |
Nano-scaled HTCC/PVA nanofibers (100–200 nm) were developed, having the potential to adsorb mammalian virus porcine parvovirus (95%). The developed system followed Freundlich isotherm and showed fast adsorption kinetics (pseudo first order), which suggested the formation of efficient filter material for the purification of water |
[57] |
|
Doxorubicin, poly (L-lactide-coD, L-lactide) and QCh |
Doxorubicin embedded poly (L-lactide-coD, L-lactide) mats were modified with QCh to enhance anti-proliferative activity. |
Developed mats were evaluated against human breast carcinoma cell lines (MCF-7) and exhibited reduced cell viability and amplified antiproliferative activity. Fluorescent microscopy revealed that the presence of QCh induced apoptosis, which was the primary mechanism of MCF-7 cell death. |
[58] |
|
2,3-Epoxy-propyl trimethyl ammonium chloride |
QCh fibres were designed using 2,3-epoxypropyl trimethyl. Ammonium chloride following ring open reaction to modify antibacterial and liquid absorption capacity. |
Outcomes revealed excellent water retention capacity, modified swelling index and mechanical strength compared to bare chitosan. Superior antibacterial efficacy against S. aureus and lower cytotoxicity suggested its vital role in fabricating wound dressing materials. |
[59] |
|
Poly (lactic acid), QCh |
Stereo complex crystallite (SC) membrane containing poly (lactic acid) QCh were employed to design disinfectant wound dressing material. |
The enhanced thermal and mechanical properties of developed SC membrane owing to restricted mobility of lactide chains. They have better wound healing capacity (100% in 15 days). This biomass-based membrane was multifunctional as it has antioxidant, antibacterial and wound healing efficacy. |
[60] |
|
Silica coated poly (vinylidene) fluoride and QCh |
High-performance anion exchange silica-coated (vinylidene) fluoride along with QCh nanofibrous membrane were designed. |
The surface of silica-coated poly (vinylidene) fluoride was grafted with quaternized chitosan to pursue dual action, i.e., ion exchange and strong reinforcement substrate. QCh-impregnated nanofibers showed superb mechanical strength (11.9Mpa). Adorned positive charges created channel-like ion transport channels that could efficiently serve as anion exchange membrane. |
[61] |
|
Objective |
Components |
Research Highlights |
References |
|---|---|---|---|
|
Multifunctional QCh-based polyacrylamide hydrogel was developed that contained hemostatic and skin adhesive properties |
QCh, Matrigel-polyacrylamide |
The developed hybrid hydrogel had a three-dimensional microporous integrity and exhibited high mechanical strength and good adhesiveness with low toxicity. The outcomes from the histology study demonstrated improvement in wound healing, collagen deposition, and stimulation of skin adnexal regeneration. The developed QCh-based antibacterial hydrogel demonstrated promising potential for designing wound dressing materials. |
[63] |
|
Dual crosslinked QCh-clindamycin loaded hydrogel was prepared to manage methicillin-resistant S. aureus (MRSA) bacteria |
QCh, clindamycin |
The developed nanocomposite-embedded hydrogel withstood sufficient mechanical and injectable efficiencies. The system responded on variable pH that enabled maximum interaction with MRSA bacteria (90% killed) in acidic conditions and overcame the antibiotic resistance challenge. |
[64] |
|
A novel wound dressing-based injectable hydrogel was designed employing QCh and PLEL (PLEL-nBG-QCS-C) hydrogel to promote angiogenesis. |
QCh and PLEL [Poly (D, L-lactide)-poly (ethylene glycol)-poly (D,L-lactide)] and bioactive glass |
PLEL hydrogels preloaded with bioactive glass (CaO-SiO2-P2O5) could efficiently seal the broken skin and increase the cure rate of wounds. Additionally, they were thermosensitive, tissue adhesive, and antibacterial. |
[65] |
|
QCh-based timolol maleate thermosensitive hydrogel was prepared for improved ophthalmic disorders. |
Timolol maleate, Sodium hydrogen carbonate, QCh |
The developed transparent thermosensitive hydrogel presented desirable porosity, swelling index, and biodegradability. The addition of sodium hydrogen carbonate enabled enhanced thermosensitivity to the system. In vitro drug release revealed the initial burst release in early hours followed by controlled release of timolol maleate for a week. This supported the potential use of the developed hydrogel for glaucoma management. |
[66] |
|
Dopamine-gelatin-crosslinked QCh injectable hydrogel was prepared to localize delivery for the combat of Parkinson and associated inflammation as well. |
Dopamine, QCh, Metronidazole, gelatin |
The formulated injectable hydrogel exhibited sufficient rheological parameters. The cytocompatibility of hydrogel revealed the cell viability and proliferation of L929 fibroblast cells. In vitro study exposed localized release of both dopamine and metronidazole. |
[67] |
|
QCh-based pH-sensitive veterinary hydrogel vaccine for improved cellular and humoral responses. |
QCh, MontanideTM ISA206 and glycerophosphate |
The developed hydrogel was biocompatible, safe, and had efficiencies to adsorb inactivated porcine reproductive and respiratory syndrome virus. Moreover, the system ruled out the downsides of mineral oil side effects and encouraged immunogenicity. |
[68] |
|
Development of NQC-loaded thermostable and multifunctional hydrogel |
N-quaternized chitosan (NQC), poly vinyl alcohol, glutaraldehyde |
Different hydrogels on varying concentration of NQC and PVA were designed to modify metal ion uptake, swelling capacity, compatibility, and antibacterial efficacy. |
[69] |
|
Objective |
Components |
Research Highlights |
References |
|---|---|---|---|
|
Ketoconazole was entrapped in QCh NPs for superior antifungal activity |
Ketoconazole, QCh, sodium triphosphate |
Nanoscaled KCZ-QCSNPs displayed superb entrapment efficiency (~90%). Performed tube dilution method revealed preeminent antimicrobial activity. |
[75] |
|
QCh derivative ‘HTCC’ NPs were embedded in various fabric materials to evaluate antimicrobial efficacy. |
HTCC, cotton fabric, polyester, polyacrylic acid |
The developed HTCC nanoparticles embedded in cotton fabric exhibited superior antimicrobial action against Fusarium oxysporum and Bacillus subtilis compared to polyester and mixture of cotton. |
[76] |
|
Anthrax vaccine adjuvant containing Fucoidan-HTCC nanoparticles were developed to improve rapid induction of immunity |
Sulphated polysaccharide (Fucoidan, FUC) and HTCC |
An active complexation between opposite-charged FUC and HTCC was conducted through varying their mass ratio. MTT assay on L929 or JAWS dendritic cells evaluated low cytotoxicity, improved cellular internalization and high cell viability. Combination of FUC-HTCCNPs and anthrax vaccine adsorbed (AVA) significantly improved magnitude of cellular/humoral immunity and mice survival rate compared to administration of AVA alone. |
[77] |
|
Nanoparticles containing N-2-HTCC and N,O-CMC encapsulated vaccine antigens (IBV/H120) were developed for significant increments in lymphocytes, interleukins, and interferon in chicken |
N-2-HTCC, N,O-carboxy methyl chitosan (CMC), infectious bronchitis virus (IBV)/H120 and Newcastle disease virus (NDV) |
The developed nanoparticles, i.e., N-2-HTCC-CMC/NDV/IBV, predicted great stability and low cytotoxicity on storing at 37 °C for 3 weeks. In vivo assay on chicken revealed sustained release of both NDV and IBV with enhanced release of IgG and IgA that facilitated the proliferation of immune modifiers in chicken body. The developed QCh-based NPs showed the potential to combat respiratory diseases in chicken. |
[78] |
|
Ecofriendly QCh derivative HTCC nanoparticles were designed to increase the durability and microbial resistance of Antheraea pernyi silk fabric. |
HTCC and 1,2,3,4 butane tetracarboxylic acid, sodium hypophosphite |
The conventional dip-and-dry-cure method was applied to evaluate silk fabric durability (A. pernyi). Wrinkle resistance, microbial resistance (against S. aureus and E. coli) and shrinkage resistance were observed even after washing A. pernyi silk fabric more than 50 times. |
[79] |
|
5-flurouracil (5-FU) embedded HTCC NPs developed for improved entrapment efficiency and in vitro release |
5-FU, HTCC, sodium tripoly-phosphate (TPP) |
5-FU/HTCC NPs were prepared through ionic gelation method via electrostatic interaction between positive-charged HTCC and negative-charged TPP. Encapsulated 5-FU exhibited controlled release profile in pH 7.4 buffer. |
[80] |