1000/1000
Hot
Most Recent

Different variables were tuned in a series of organic semiconductors synthesized in the group of José L. Segura, such as the planarity and the length of their π-conjugated backbones, the topology and energy levels of the frontier molecular orbitals (HOMO and LUMO) and their molecular dipole moments. Studies carried out in the group of Rocío Ponce-Ortiz show that the tuning of these properties can be connected with the microstructure properties observed by atomic force microscopy (AFM) and X-ray diffraction (XRD) in thin films as well as with the performances in organic field-effect transistors (OFETs).
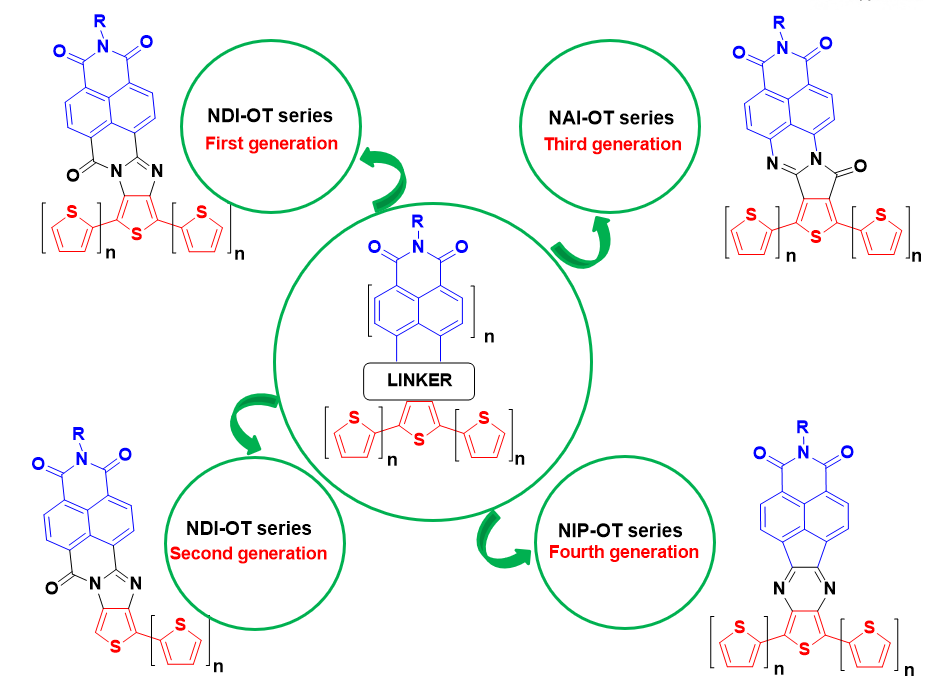
Our first approach to the desired hybrids connected through a rigid and conjugated linker involved the syntheses of thiophene-fused rylenimide derivatives via imidazole linkers. This first generation of organic semiconductors was obtained by condesation between suitable a diaminooligothiophene unit and the corresponding monoanhydride monoimide derviative (Scheme 1)
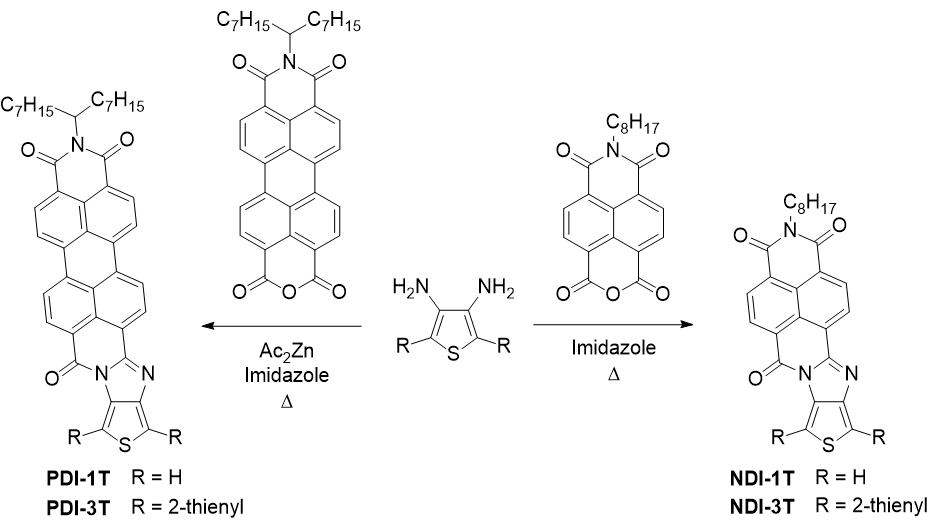
Scheme 1. Synthesis of NDI and PDI derivatives
Electrochemical characterization of these assemblies was carried out by using cyclic voltammetry measurements. Both reduction and oxidation processes were observed in all the naphthalene- and peryleneimide derivatives (Table 1). From these data, LUMO and HOMO energies could be estimated using standard approximations (Figure 2 and Table 1) [16,17], being -3.79/-5.83 eV for NDI-1T and -3.87/-5.55 eV for the NDI-3T analogue. A slight stabilization of the LUMO and a remarkable destabilization of the HOMO energy level around 0.3 eV, is observed when the oligothiophene fragment is extended. This electrochemical band gap reduction is consistent with the band gap compression observed in the UV-vis spectra. Further reduction of the electrochemical band gap can be achieved by extending the π-system of the rylenimide unit through replacement of the naphthalimide moiety by the larger perylenimide analogue. Thus, LUMO/ HOMO energies were estimated to be -3.88/-5.76 eV for PDI-1T and -3.89/-5.55 eV for PDI-3T. The extension of the oligothiophene moiety does not significantly alter the LUMO level while produces a destabilization of the HOMO level.
Table 1. Electrochemical potentials versus SCE in CH2Cl2 (referenced to Fc/Fc+ couple) of NDI-1T, NDI-3T, PDI-1T, PDI-3T and NDI-T-Ph-T and Frontier Molecular Orbital Energy levels estimated from CV data.
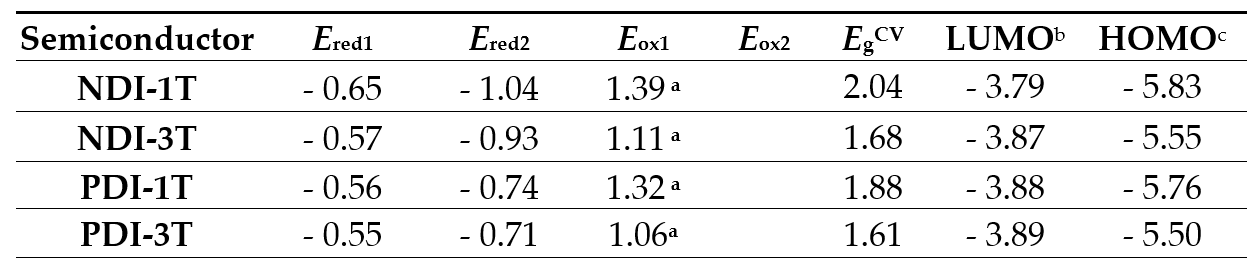
a Irreversible. b LUMO energy levels estimated vs vacuum level from ELUMO = -4.44 eV - e Ered1. c Estimated from HOMO = LUMO – EgCV. EgCV = electrochemical gap.
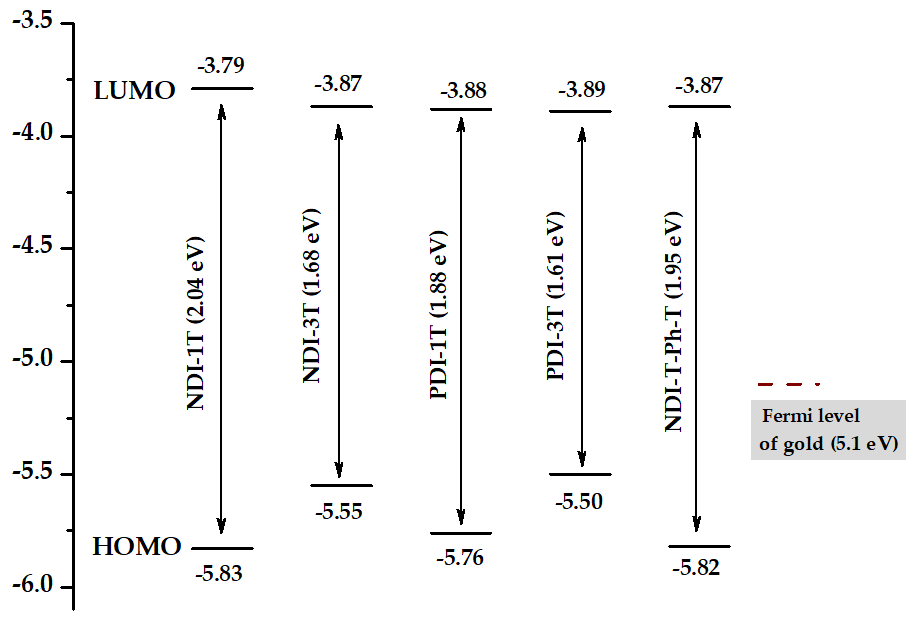 Figure 2. HOMO and LUMO energy levels for NDI-3T, NDI-3T, PDI-1T, PDI-3T and NDI-T-Ph-T
Figure 2. HOMO and LUMO energy levels for NDI-3T, NDI-3T, PDI-1T, PDI-3T and NDI-T-Ph-T
In order to evaluate the electrical performances of these first-generation of oligothiophene-rylenimide semiconductors, films were grown and applied to top-contact-bottom gate (TC/BG) field effect transistors in the group of Prof. Rocío Ponce-Ortiz at the Malaga University. The films were obtained either by vapor deposition or solution processed onto Si/SiO2 substrates pre-treated with HDMS or OTS. The best degree of crystallinity was found for NDI-1T because of the formation of cofacial π-conjugated planes aligned to the Si/SiO2 surface with a calculated tilt angle of 61,2°. In contrast, NDI-3T thin films resulted into an amorphous material probably due to the lack of planarity caused by sulfur-oxygen repulsion interaction which forces the disruption of the oligothiophene planarity. Although they are less crystalline than NDI-1T, the presence of sharp Bragg reflections in the X-ray diffractograms of PDI-1T and PDI-3T evidenced the existence of a certain degree of crystallinity in the perylene derivatives.
TC/BG field-effect transistors properties were evaluated in terms of charge carrier mobility (μ), current on-off ratio (ION/IOFF) and threshold voltage (VT) (Table 2). The best charge transport characteristics were found for NDI-1T when vapor-deposited films were grown at 110 °C on Si/SiO2 surfaces treated with HMDS, with electron mobilities reaching values of 0.35 cm2V-1s-1. These good results regarding NDI-1T could be explained in terms of high crystallinity, good molecular packing in well-connected grains as observed in XRD and AFM analyses. The smaller grain sizes and lower crystallinity observed for PDI-1T with a more extended π-conjugated system accounts for the slightly lower electron mobility of 0.1 cm2V-1s-1 measured for this system. Regarding naphthalimide-based assemblies, the introduction of the phenylene moiety in NDI-T-Ph-T enhanced the crystallinity and the presence of well-defined and well-connected smaller grains, allowing mobilities of around 0.1 cm2V-1s-1 for electron transport. Finally, the introduction of catenated thiophene units in both NDI-3T and PDI-3T decreases their performances in the devices in around two orders of magnitude in agreement with the analyses of AFM images and XRD patterns. In contrast with the analogue with one thiophene unit (NDI-1T), the introduction of additional thiophene moieties in NDI-3T produce a twist in the oligothiophene backbone thus hindering an efficient packing and therefore causing a decrease in the crystallinity of the films. As a result, electron mobilities of only 10-4 cm2V-1s-1 can be measured for these assemblies. In comparison with the naphthalimide containing assembly NDI-3T, films made from the perylenimide containing analogue, PDI-3T, are more crystalline, having larger grains that allow better charge carrier mobilities of up to 10-3 cm2V-1s-1. Solution processed OTFT transistors were also fabricated, and their morphologies and crystal structures were evaluated. The results obtained for these semiconductors were in good agreement with the new morphologies obtained for each one and, in the case of PDI-3T, an electrical enhancement was found in comparison with the vapor-deposited thin-film transistors.[10]
Table 2. OTFT Electrical data for Vapour-Deposited films of NDI-1T, NDI-3T, PDI-1T, PDI-3T and NDI-T-Ph-T measured under vacuum on Si/SiO2 substrates.a
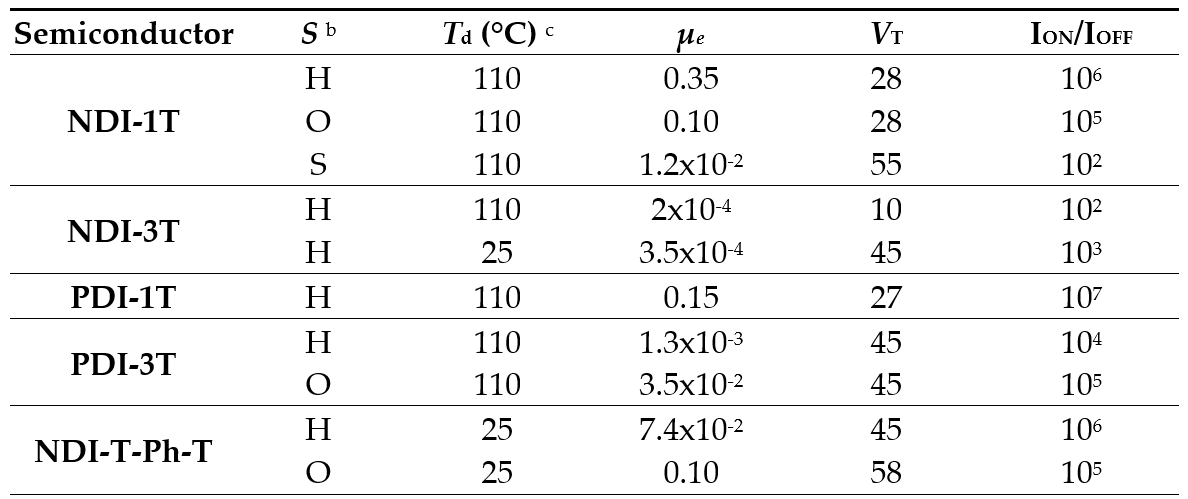
a Electron carrier mobility (μ) is given in cm2V-1s-1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on untreated Si/SiO2 substrates (S), hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature
Motivated by the above results,[10] a novel family of naphthalimide-oligothiophene assemblies[11] (second-generation NDI-nT) was designed with the aim to avoid the above described skeletal distortion (Figure 3).
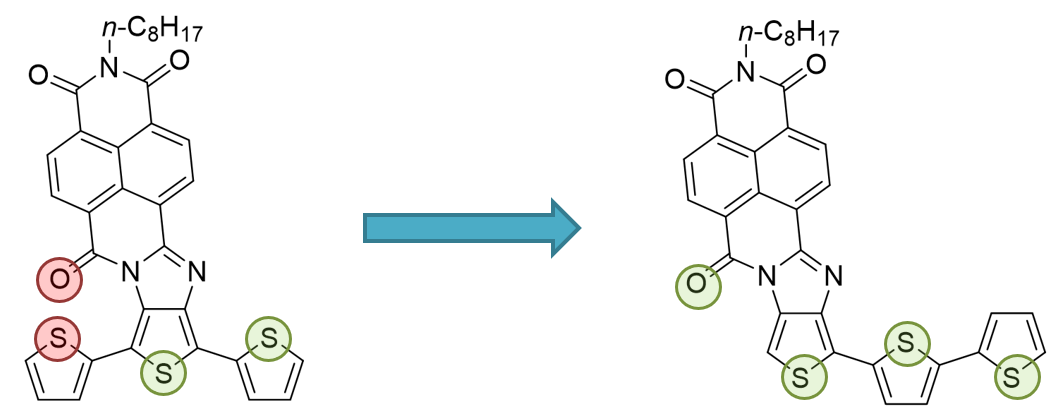 Figure 3. Schematic representation of oxygen-sulfur repulsive interaction (highlighted in red) in the first generation of NDI-oligothiophene derivatives and how it is suppressed in the second generation.
Figure 3. Schematic representation of oxygen-sulfur repulsive interaction (highlighted in red) in the first generation of NDI-oligothiophene derivatives and how it is suppressed in the second generation.
In order to obtain this second generation of oligothiophene-naphthalimide assemblies, a linear synthetic approach was followed (Scheme 2) instead of the convergent one used previously.[10][12][13] Thus, a monobrominated thiophene-naphthalimide derivative (NDI-1T-Br, Scheme 2) was used as starting material. Stille cross-coupling reactions between NDI-1T-Br and the corresponding monostannane derivatives were carried out in order to obtain the target molecules NDI-2T, NDI-3Tp and NDI-4T in good yields.[11] As depicted in Scheme 2, the steric repulsion between the oxygen and sulfur heteroatoms is absent in these new organic semiconductors thus preventing the skeletal distortions. Additionally, NDI-5T (Scheme 2) was synthesized for comparison purposes given that it combines a fragment with the extended π conjugated structure of NDI-3Tp and also a fragment with the steric hindrance of NDI-3T. NDI-5T could be obtained by Suzuki cross-coupling reaction between NDI-1T-2Br and the 2,2′−Bithiophene-5-boronic acid pinacol ester.
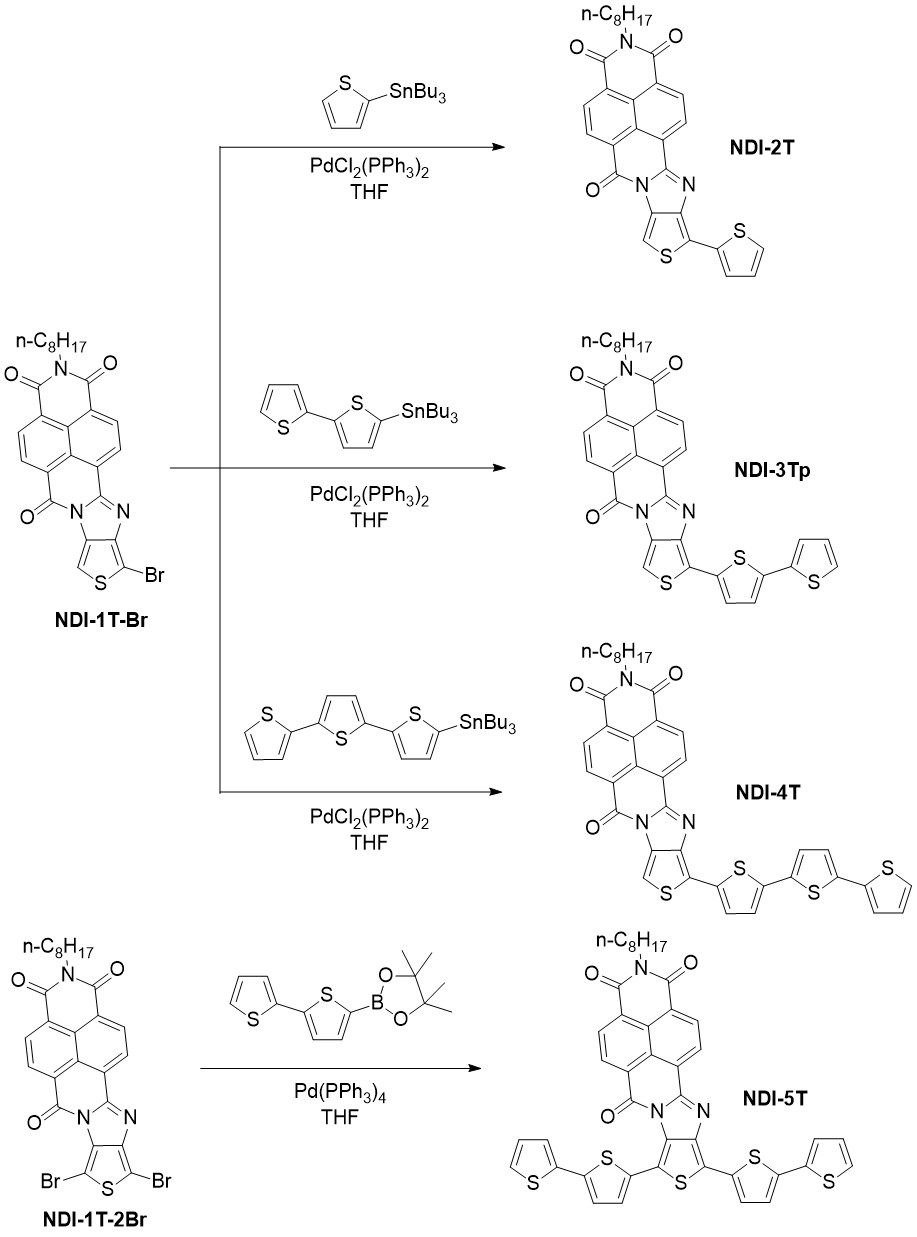 Scheme 2. Syntheses of NDI-nT materials.
Scheme 2. Syntheses of NDI-nT materials.
The HOMO levels of NDI-4T and NDI-5T approaches the Fermi level of Au thus improving the hole injection in electronic devices.
It is also worth noting that the band gap of the planar NDI-3Tp is 0.1 eV smaller than that of the NDI-3T analogue with a distorted structure (Figure 4). This energy readjustment, in conjunction with other morphological and electronic factors, is probably the origin of the ambipolar behavior shown by NDI-3Tp.
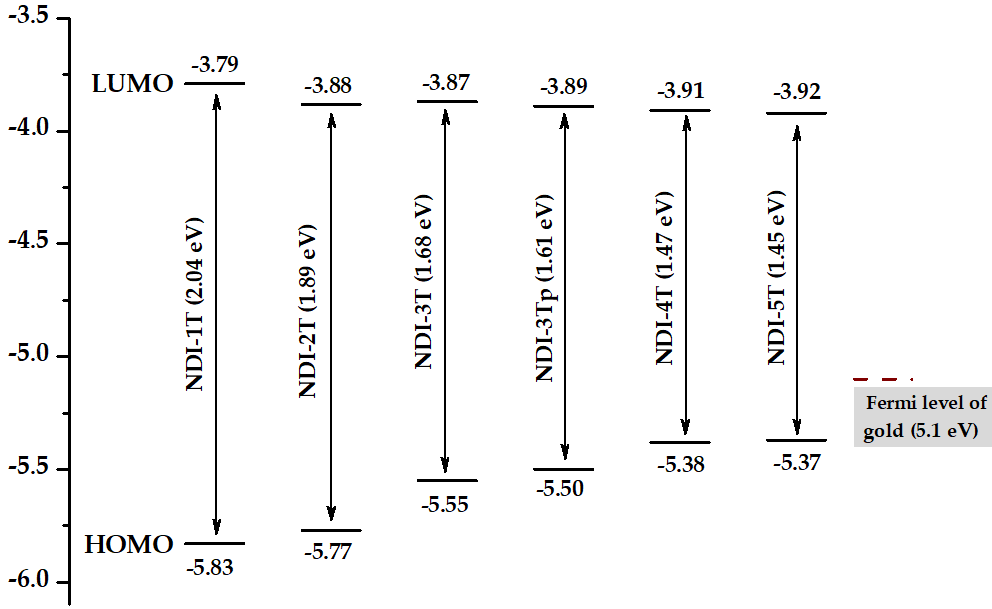 Figure 4. HOMO/LUMO energy levels derived from the electrochemical measurements. Energy gaps are shown in brackets.
Figure 4. HOMO/LUMO energy levels derived from the electrochemical measurements. Energy gaps are shown in brackets.
Thin films of these NDI-nT semiconducting materials were processed from solutions or obtained by sublimation. X-ray analyses of the thin films show that crystallinity is much more pronounced in those films obtained by sublimation. The most remarkable characteristics of the FET performances of these semiconductors were investigated in TC/BG OFETs (Table 3). The shortest derivative, NDI-2T, only shows electron mobilities up to 2.6x10-2 cm2V-1s-1. The fact that NDI-2T only exhibit n-type mobility values is consistent with the similarity of its HOMO energy level and that of NDI-1T. In contrast, ambipolarity was found for NDI-3Tp and NDI-4T. This ambipolar behavior could be related to: 1) the lack of skeletal distortions that allow close π-π stacking interactions and lower reorganization energies and 2) the presence of longer conjugated oligothiophene moieties with increased π conjugation lengths that allow a destabilization of the HOMO energy levels which approach the Fermi level of Au. Interestingly, FETs fabricated using NDI-5T exhibit ambipolar transport behavior with balanced electron and hole transport mobilities of approximately 10-5 cm2V-1s-1. The study demonstrates that the absence of ambipolarity is mainly due to the disruption in the π conjugation while a decrease in the carrier mobility is induced by the skeletal distortions. Furthermore, there is a good correlation between the charge transport properties, the film microstructures and the ordered packing of non-distorted structures.
Table 3. OFET Electrical data for Vapour-Deposited films of NDI-2T, NDI-3Tp, NDI-4T and NDI-5T measured under vacuum on Si/SiO2 substrates. Average field-effect mobilities are shown.a
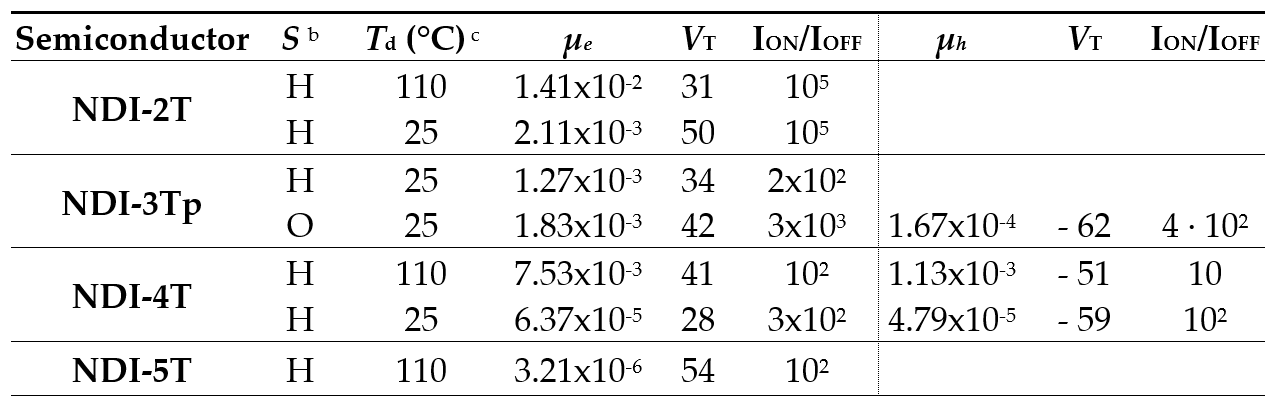
a Electron carrier mobility (μ) is given in cm2V-1s-1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature.
The lessons learned from the first series of oligothiophene–naphthalimide assemblies allowed us to conclude that ambipolar semiconductors with these kinds of systems can be obtained by (i) connecting the donor and acceptor moieties through conjugated rigid linkers, (ii) avoiding steric hindrance and skeletal distortions and (iii) extending the effective π-conjugated length of the oligothiophene moiety.
In the search for new low-bandgap materials in which facile hole and electron injection are possible from a single type of electrode, we designed another new family of oligothiophene–naphthalimide assemblies, NAI derivatives (Figure 5) that involves the inversion of the amidine linkage connections between the oligothiophenes and the naphthalimide in order to prevent steric interactions (Figure 5).[14]
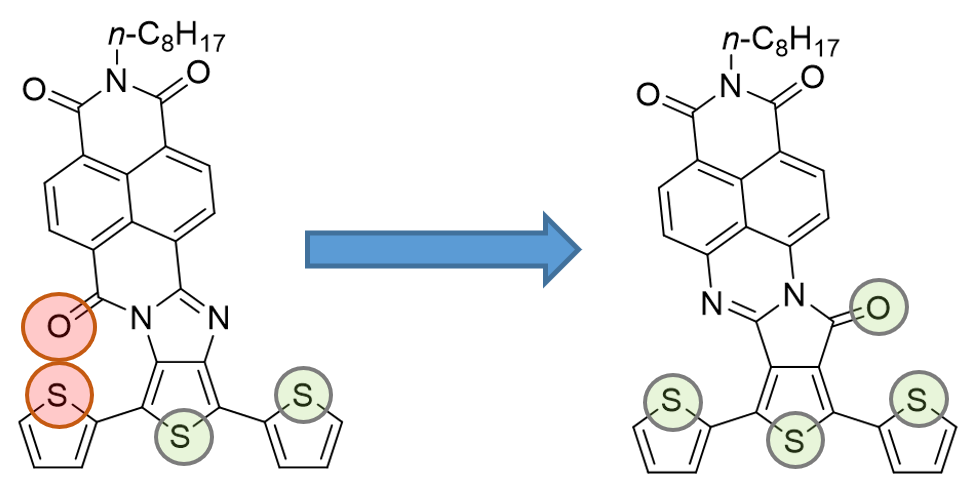 Figure 5. Illustration of the oxygen–sulfur interaction in NDI-3T and NAI-3T derivatives.
Figure 5. Illustration of the oxygen–sulfur interaction in NDI-3T and NAI-3T derivatives.
As it is depicted in scheme 3, for the syntheses of the NAI derivatives, naphthalimide moieties endowed with amino functionalities and a thiophene derivative endowed with anhydride functionality were used as starting materials. Through a condensation reaction between the mentioned monomers, the dibrominated NAI-1T-2Br derivative can be obtained. Further reaction of NAI-1T-2Br by Stille cross-coupling reactions with the corresponding stannylated thiophene or bithiophene yield the target molecules NAI-3T and NAI-5T. In terms of the molecular structure, the inversion in the orientation of the carbonyl group in the imidazole unit provides more planar semiconductors, in which the O···S repulsive interaction is deliberately avoided in contrast with that observed in the first generation of oligothiophene–naphthalimide assemblies with noninverted amidine linkers.[10][11]
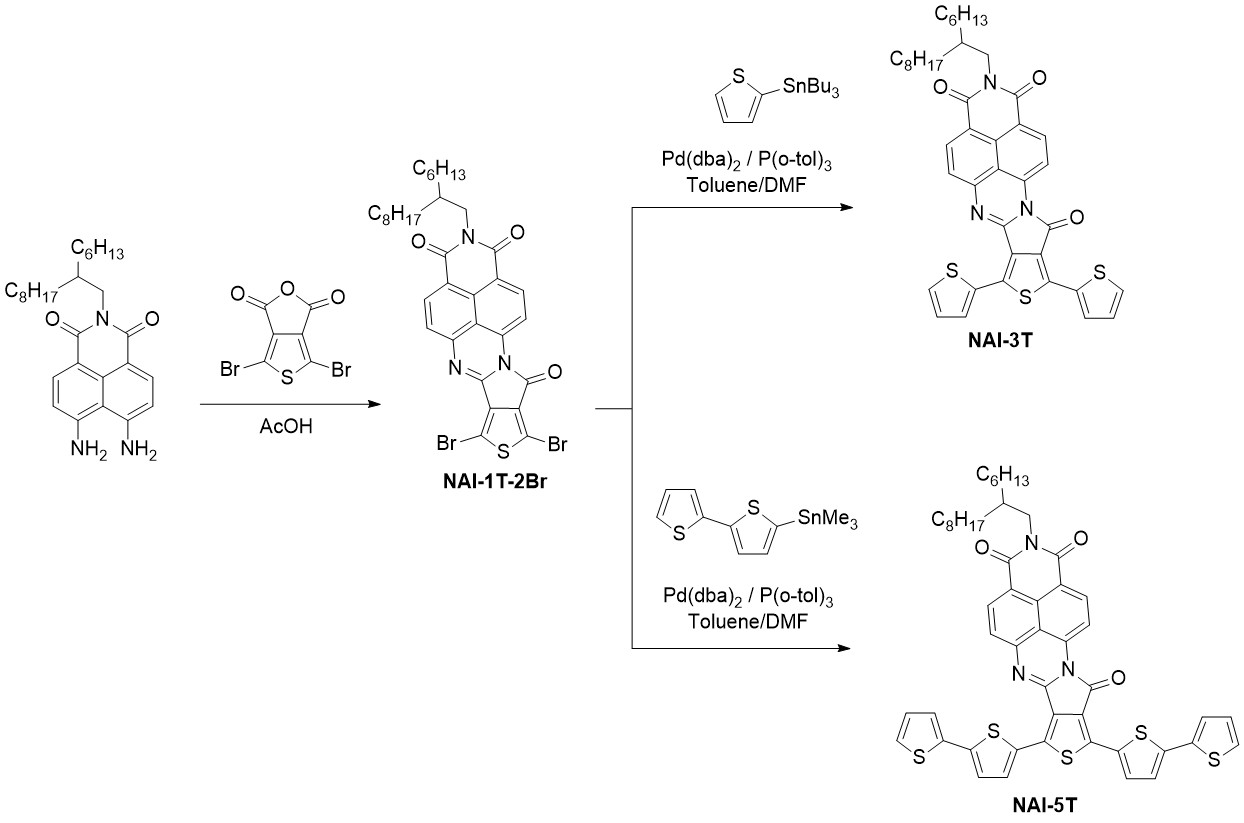
Scheme 3. Syntheses of the fourth generation of oligothiophene–naphthalimide assemblies.
Cyclic voltammetry measurements for both NAI-3T and NAI-5T present oxidation and reduction processes. From these values, the HOMO and LUMO energy levels could be estimated (Figure 6). The LUMO energy levels estimated for the NAI derivatives are significantly destabilized in comparison with those of the NDI analogues, while the HOMO energy levels of both NAI-3T and NAI-5T are more stabilized than those corresponding to their NDI counterparts.[10][11][15]
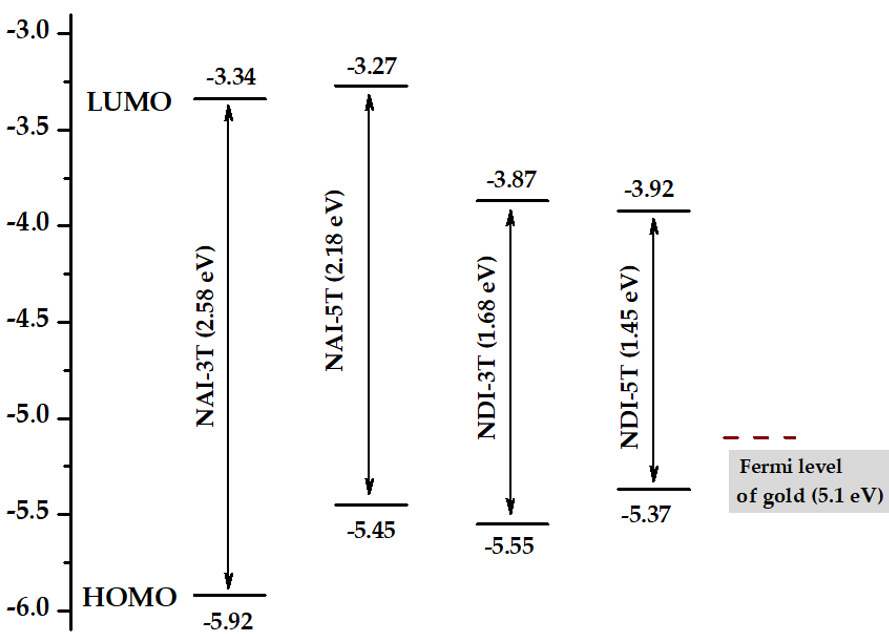
Figure 6. HOMO/LUMO energy levels for oligothiophene–naphthalimide assemblies based on NDI and NAI.
Finally, in order to evaluate the electrical properties of these new semiconductors, TC/BG field-effect transistors were fabricated by vapor deposition. The OFET performance obtained for the vapor-deposited films is summed up in Table 4. In this semiconductor series, p-type and ambipolar transport properties were found for NAI-5T and NAI-3T, respectively. In the shorter member of the series, the electron mobilities are similar to that of the NDI analogues, around 1.9 × 10−4 cm2 V−1 s−1, with interesting balanced values for hole mobilities of 2.0 × 10−4 cm2 V−1 s−1 at 25 and 110 °C. Although the outputs for NAI-5T show clear ambipolarity, only hole-charge transport values of 5.75 × 10−5 cm2 V−1 s−1 were ultimately measured. The better hole mobility values obtained for NAI-5T in comparison with the parent NDI-5T could be related to the HOMO delocalization over the whole molecule, which allows better orbital overlapping between molecules.
Table 4. OFET electrical data for vapor-deposited films of NAI-3T and NAI-5T measured under vacuum on Si/SiO2 substrates. Average field-effect mobilities are shown a.
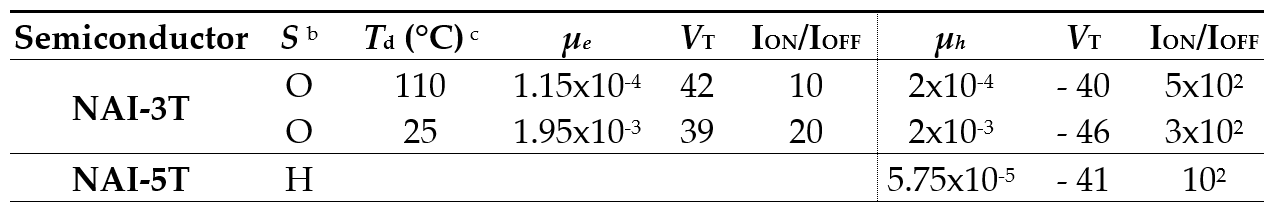 a Electron carrier mobility (μ) is given in cm2V-1s-1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature.
a Electron carrier mobility (μ) is given in cm2V-1s-1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature.
As it was previously shown, avoiding steric hindrance and skeletal distortions in naphthalimide-oligothiophene assemblies plays a crucial role in the search of ambipolarity. For that reason, we designed a fifth generation of naphthalimide-oligothiophene assemblies in which flat and rigid pyrazine units are used as conjugated linkers (Figure 7).
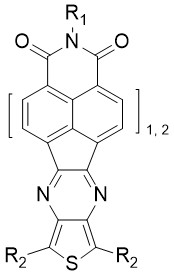
Figure 7. General structure of the fifth generation of oligothiophene-naphthalimide assemblies
The pyrazine-based materials can be obtained by condensation reactions between naphthalene- (NID) or peryleneimides (PID) endowed with 1,2-diketone functionalities, and the corresponding diaminothiophene derivatives (Scheme 4).[16][17]
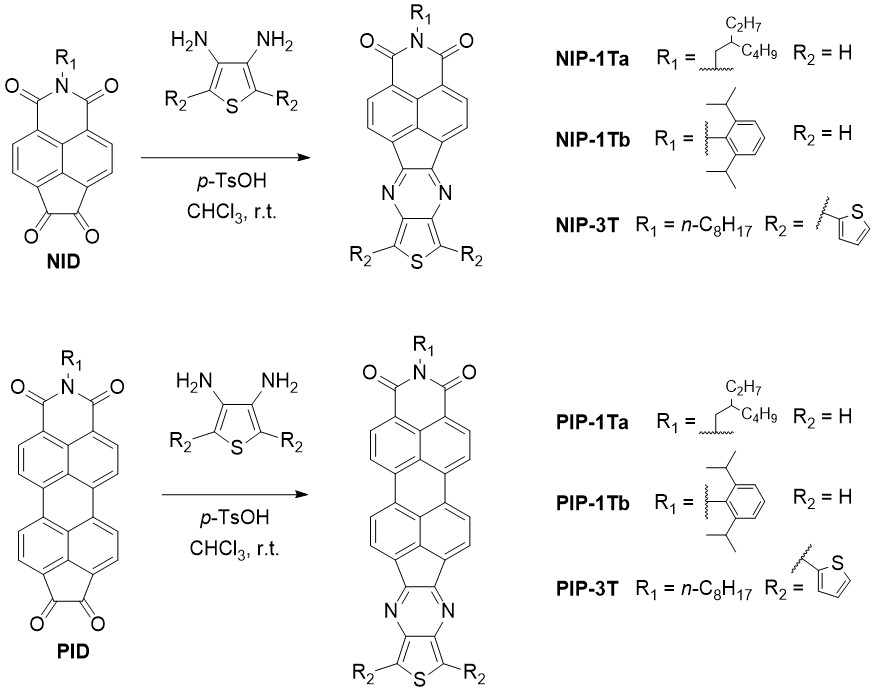 Scheme 4: Syntheses oligothiophene-naphthalimide (NIP) and perylenimide (PIP) assemblies with pyrazine linkers.
Scheme 4: Syntheses oligothiophene-naphthalimide (NIP) and perylenimide (PIP) assemblies with pyrazine linkers.
In order to study the electrochemical properties of these planar pyrazine-based assemblies, cyclic voltammetry experiments were carried out, and from the obtained electrochemical values, frontier molecular orbital energies could be estimated. As we previously described, oligothiophene chain catenation had an important effect over the HOMO energy, showing destabilized values as the oligothiophene is growing, whereas LUMO energies were barely altered.[11][15] Lowest unoccupied molecular orbital energy levels depend on the extension of the rylenimide units. Thus, the estimated LUMO values for NIP-1T and PIP-1T are -3.40 eV and -3.49 eV respectively. Regarding to the oligothiophene counterpart, an increment in the number of the thiophenes has negligible effect in the LUMO level, while this chemical modification affects clearly the HOMO energy levels, and therefore the optical band gap. The HOMO energy level estimated for PIP-1T (-5.34 eV) is significantly estabilized in comparison to that estimated for PIP-3T (-4.75 eV). The destabilization of the HOMO observed for PIP-3T is in good agreement with the extension of the thiophene backbone and with the absorption spectra. Therefore, in these pyrazine-based semiconductors, tuning the HOMO-LUMO bandgap can be achieved by two approximations: (i) by extending the rylenimide core without modifying the donor moiety and (ii) through the increment in the length of oligothiophene backbone. Comparison between the HOMO and LUMO energy levels of the NIP and PIP semiconductors and those of the NDI and PDI analogues reveals that the replacement of the imidazole linker by a rigid pyrazine heterocycle is reflected into more destabilized HOMO and LUMO orbitals, as well as in the enlargement of the band gap (Table 5).[11][17]
Table 5. Electrochemical potentials versus SCE in CH2Cl2 (referenced to Fc/Fc+ couple) of NIP-1T, NIP-3Ta, PIP-1T and PIP-3T and Frontier Molecular Orbital Energies estimated from CV data.
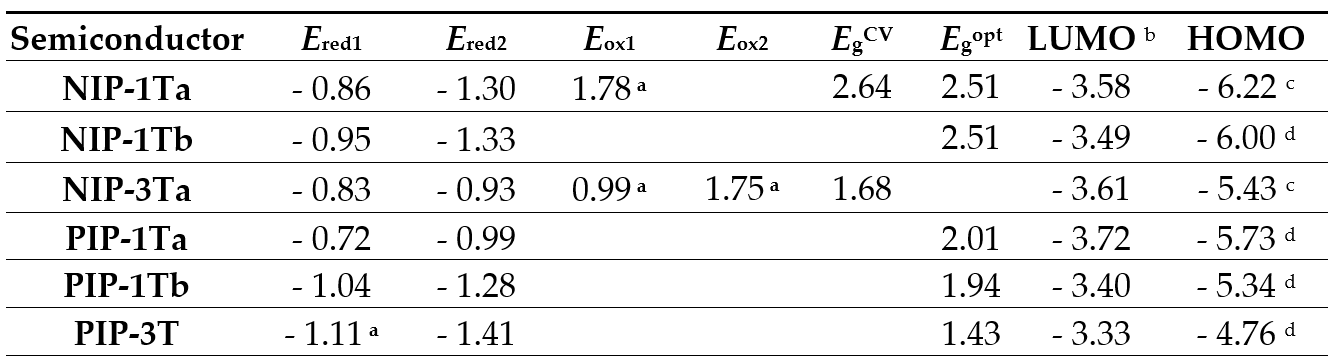
a Irreversible. b LUMO energy level estimated vs vacuum level from ELUMO = -4.44 eV - e Ered1. c Estimated from HOMO = LUMO – EgCV. EgCV = electrochemical gap. d Estimated from HOMO = LUMO – Egopt. Egopt = optical gap.
As a common evaluation of the electrical properties for these pyrazine-based oligothiophene-rylenimide derivatives, the fabrication of TC/BG thin-film organic field-effect transistors was accomplished. Concerning with the charge transport ability of these thienopyrazine derivatives, all of them showed n-type mobilities, with a best electron mobility value of 2.87·10-4 cm2V-1s-1 for NIP-1T when it was deposited at 50 °C on HMDS-treated dielectric (Table 6). NIP-3T is the only thienopyrazine derivative that show ambipolar behavior, with good balanced values for both hole and electron charge carriers. This effect is related with the high planar structure and low internal reorganization energies, enabling both electron and hole transport processes. The same behavior could be expected for PIP-3T, but only discrete n-type mobility was observed (1.2 x10-4 cm2V-1s-1). This result could be explained in terms of the formation of antiparallel π-π stackings among the molecules when they were vapor-deposited over the dielectric, restricting the formation of the D and A domains necessary for the hole and electron transporting. This aggregation behavior is in good agreement with the predicted computational study for dimeric species.[17]
Table 6. OFET Electrical data for Vapour-Deposited films of NIP-1Ta, NIP-3Ta, PIP-1T and PIP-3T measured under vacuum on hexamethyldisilazane vapor-treated Si/SiO2 substrates. Average field-effect mobilities are shown.a
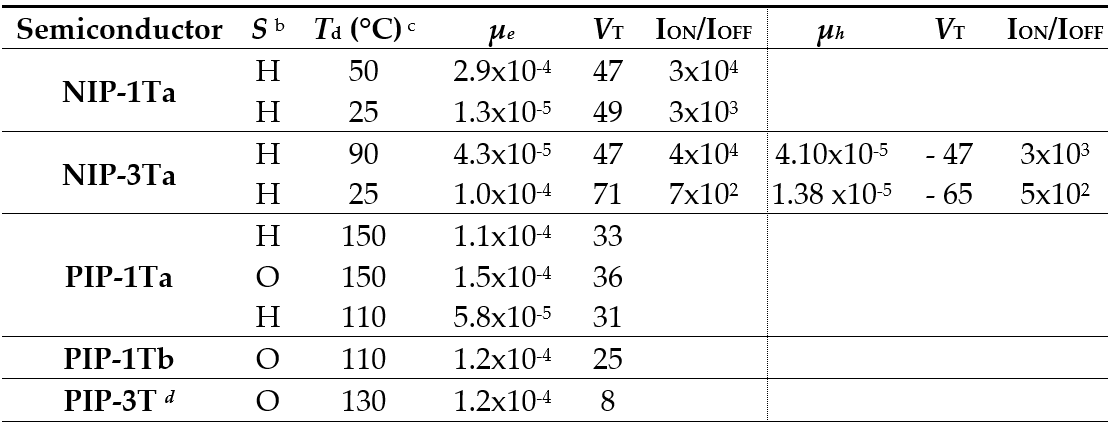
a Electron carrier mobility (μ) is given in cm2V-1s-1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature. d Solution processed film.
To further explore the possibility of tuning the performance of this type of material in (opto)electronic devices, we designed a family of oligothiophene–naphthalimide assemblies based on pyrazine linkers in which end-capped units were introduced in order to promote good packing in the molecule while the HOMO/LUMO energy levels are still suitable for semiconducting applications.[18]
These new thienopyrazine-based semiconductors are endowed with different substituents in order to promote molecular ordering, better processability and tuning of the frontier molecular orbitals. These oligothiophene–naphthalimide assemblies can be divided into different groups depending on the nature of the end-capped units introduced: (i) substituents that promote intermolecular interactions and (ii) different electron acceptors in order to modulate the HOMO/LUMO energy levels. Thus, there are three end-capped derivatives functionalized with pyrene, triisopropilsilyl and diphenylamine with a donor-acceptor-donor (D-A-D) structure (Scheme 5). On the other hand, the introduction of electron acceptor substituents via Knoevenagel-like reactions led to obtaining A2-D-A1-D-A2 semiconductors endowed with rhodanine and dicyanovinylene end groups (Scheme 6).
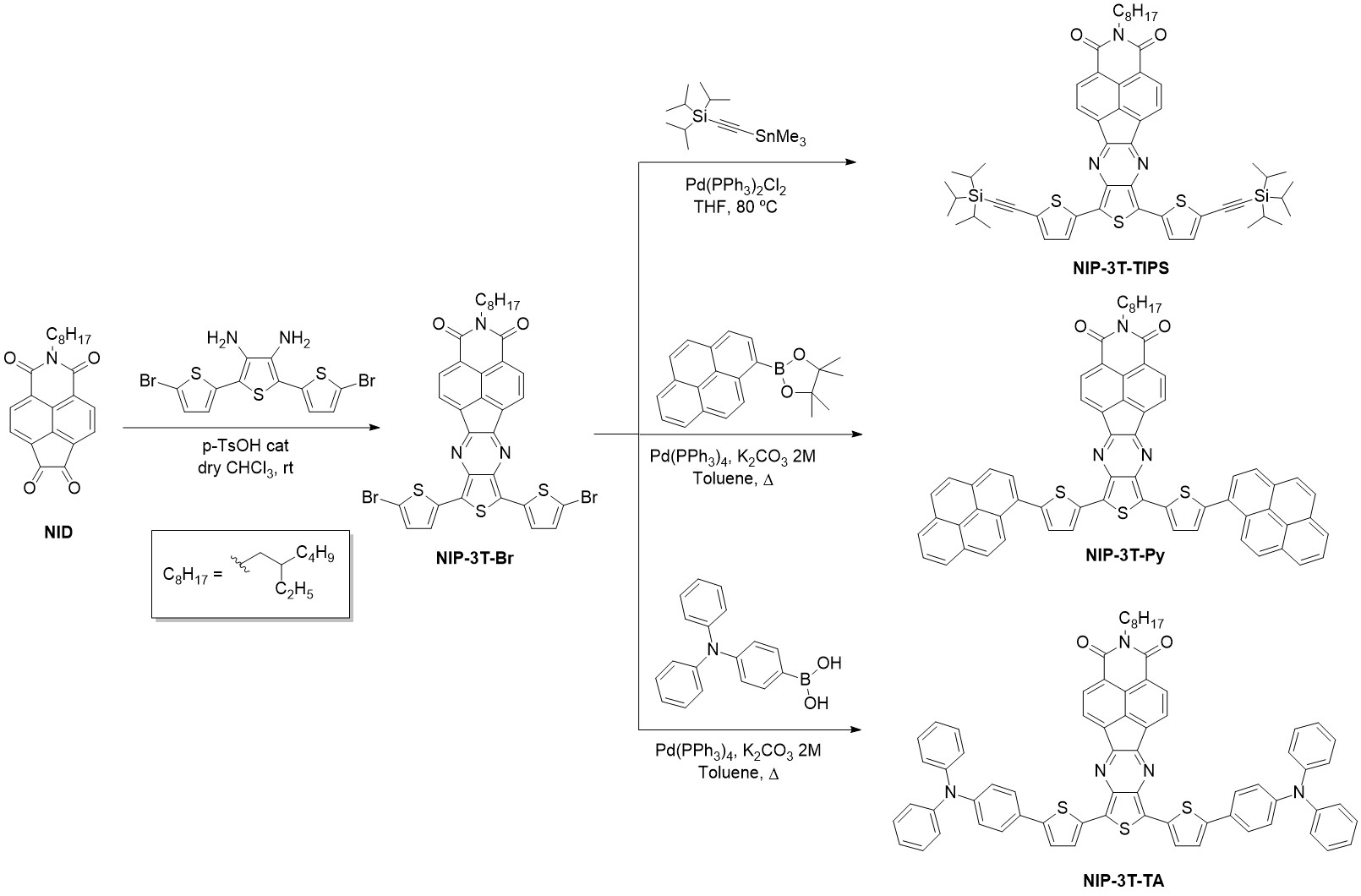
Scheme 5. Syntheses of D-A-D oligothiophene–naphthalimide assemblies.
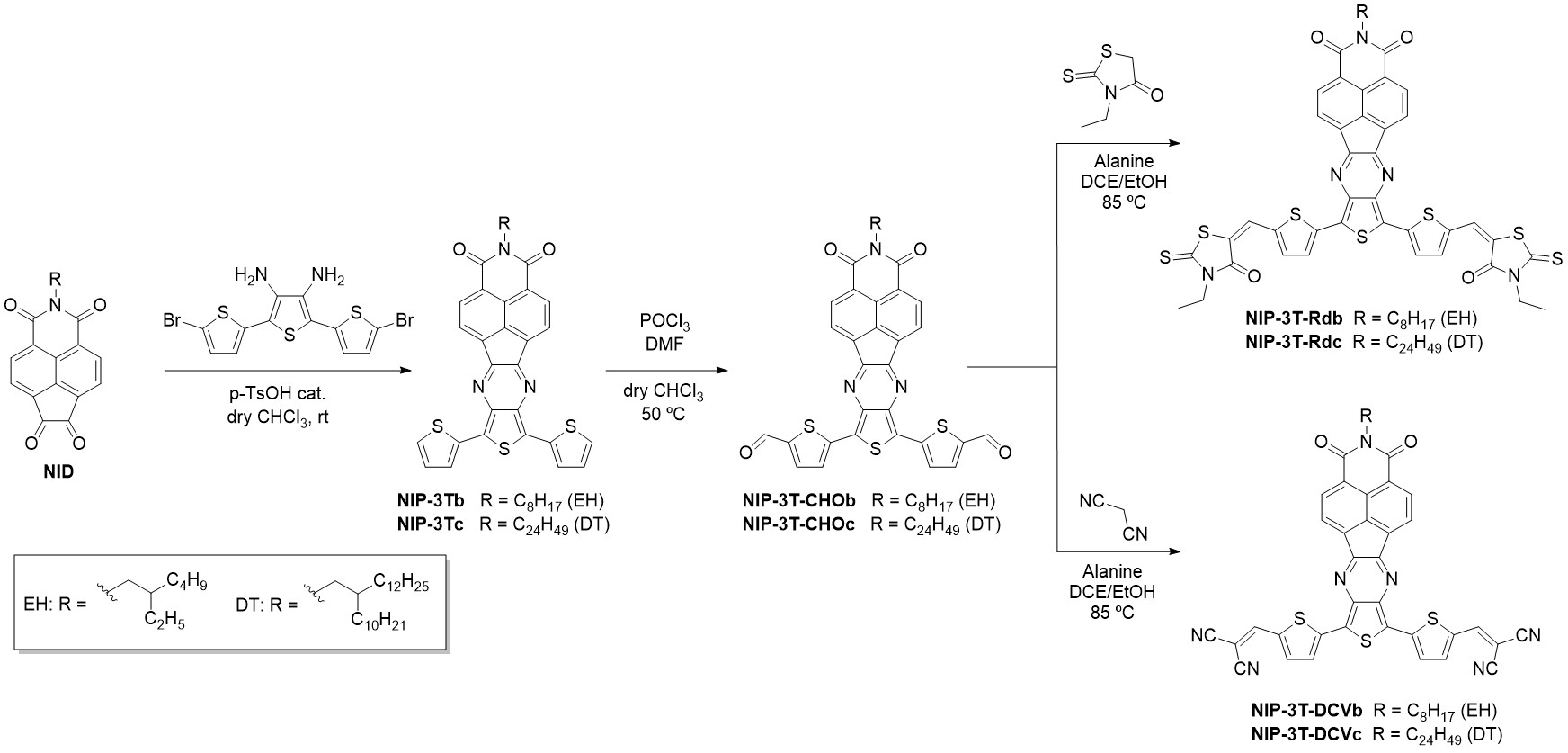
Scheme 6. Syntheses of A2-D-A1-D-A2 oligothiophene–naphthalimide assemblies.
The electrical characterization of these new semiconductors was carried out in organic field-effect transistors with TC/BG architectures. The thin films deposited by vapor deposition were also characterized by XRD and AFM techniques. As previously shown, NIP-3Tb has ambipolar characteristics, with balanced holes and electron mobility values of 4 × 10−5 cm2 V−1 s−1. The introduction of lateral substituents modifies the polarity in the charge transport properties, as it was shown for NIP-3T-TIPS, in which only p-type characteristics were found. On the other hand, when pyrene moieties are introduced laterally in the NIP-3T-Py derivative, electron mobilities of 10−2 cm2 V−1 s−1 were found, the best of all these semiconductors (Table 7). In contrast, when electron-withdrawing groups are introduced, only n-type mobilities were observed, except in the case of NIP-3T-Rdb in which ambipolarity is observed (Table 7). The different behavior observed for the end-capped oligothiophene–naphthalimide semiconductors can be also related to the limited crystallinity observed in XRD and AFM images. It is worth pointing out that, given the suitable HOMO and LUMO energy levels, as well as good processability and full absorption range even in the NIR spectra of the oligothiophene–naphthalimide assemblies endowed with electron-acceptor units, they have also recently received some attention as nonfullerene acceptors in OSCs.[18]
Table 7. OFET electrical data for vapor-deposited films of NIP-3Tb, NIP-3T-DCVb, NIP-3T-Rdb, NIP-3T-Py and NIP-3T-TIPS measured under vacuum on vapor-treated Si/SiO2 substrates. Average field-effect mobilities are shown a.
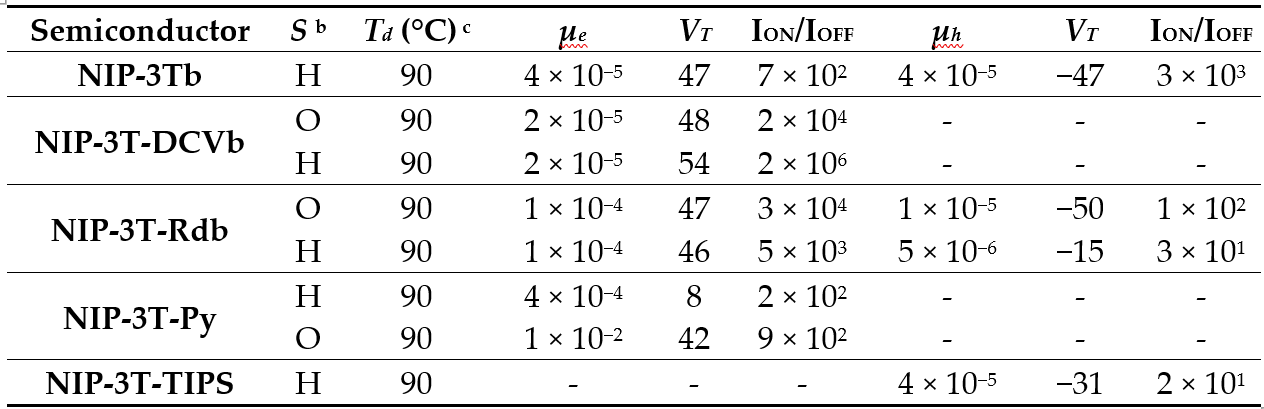
a Electron carrier mobility (μ) is given in cm2 V−1 s−1 and threshold voltages (VT) in V. b Device parameters reported are for films grown on hexamethyldisilazane vapor-treated Si/SiO2 substrates (H) or octadecyltrichlorosilane-treated Si/SiO2 substrates (O). c Deposition temperature.
The implementation of conjugated molecular assemblies into polymeric materials has proven to be an efficient strategy to develop new semiconductors for a wide variety of applications.[19][20][21][22] Among them, the development of metal-free, organic polymers as photocatalysts[23] for the removal of hazardous contaminants[24][25] has been revealed as an emerging area in the last decade.
In this regard, molecular- and polymeric-conjugated assemblies with wide absorption cross-section characteristics are good candidates as photocatalysts. Moreover, it is known that materials which combine electron donor and electron acceptor units are also interesting in photocatalysis because fast recombination processes may be inhibited. The possibilities offered by the oligothiophene–naphthalimide assemblies to efficiently tune their optical and electronic properties as well as their suitability to be functionalized at the α positions of the oligothiophene moieties make these types of assemblies good candidates to be used as monomers for the syntheses of donor-acceptor polymeric materials suitable for the photocatalytic degradation of organic pollutants in water.[26]
Poly(azomethine) network NIP-3T-ANW (Figure 6) was prepared under solvothermal reaction conditions.[27] Interestingly, this polymeric material shows high thermal stability, without noticeable weight loss up to 450 °C, in contrast with the NIP-3T molecular semiconductor in which the degradation starts at 200 °C. Furthermore, the material exhibits a good energy band alignment for the photodegradation of some organic pollutants, such as rhodamine B (RhB). Due to the above characteristics, the potential of NIP-3T-ANW as photocatalyst in the photodegradation of RhB has been investigated. It is known that RhB is stable in aqueous solution under illumination in the absence of catalyst. In contrast, when NIP-3T-ANW is added, after 120 min, almost a 90% of RhB is degraded in the aqueous solution, and no stability problems in the catalyst are detected after four catalytic cycles. For comparison purposes, the photocatalytic activity of the analogue molecular component NIP-3T has been also investigated, showing only a 55% degradation of RhB in the same time period. Thus, the incorporation of the D-A unit into the polymeric network dramatically increases its activity and allows for its recyclability without losing efficiency (Figure 8).
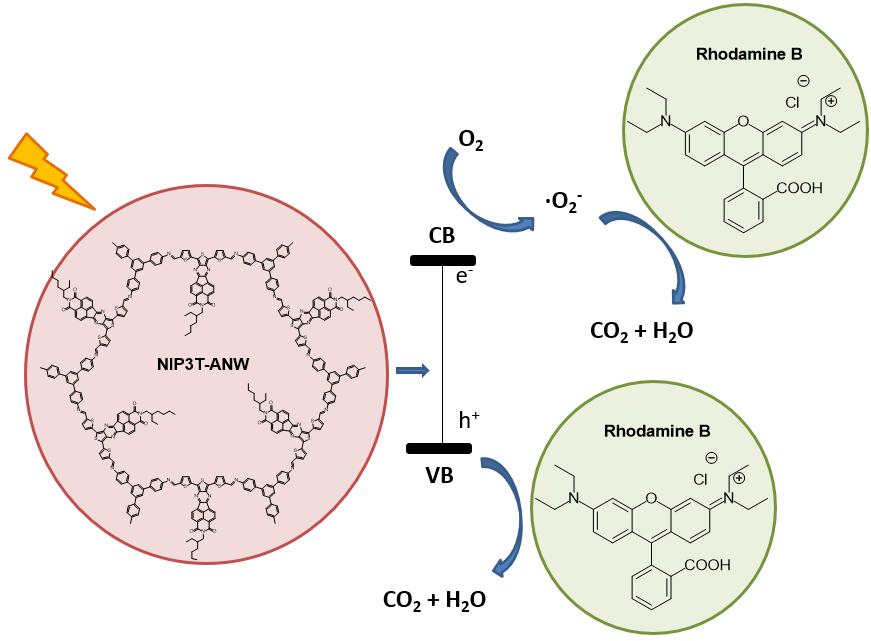
Figure 8. Structure of NIP-3T-ANW and its photocatalytical activity
In this article, we have described and compared several families of oligothiophene–naphthalimide assemblies synthesized in the group of José L. Segura in which the donor and the acceptor units are connected through different rigid heterocycles. The synthetic modifications in the molecular structure, by growing the oligothiophene or the rylenimide moieties, is translated into a precise control over the frontier molecular orbital energy levels, allowing for the segregation of the HOMO and the LUMO in the molecule, promoting better charge carrier pathways. Studies carried out in the group of Rocío Ponce-Ortiz show that the structural modifications in these molecular systems produce changes in the microstructure domains, in the crystal diffraction patterns and in the electrical performance found in the thin-film organic field-effect transistors. Moreover, it has been shown that the modification of the naphthalimide–oligothiophene central core is not the only effective way to promote order into the molecule. In fact, the introduction of lateral hydrophilic or hydrophobic chains or end-capped terminal groups also represent interesting approaches to obtain organized assemblies via supramolecular interplay, such as Van der Waals interactions or π–π stacking. Finally, the incorporation of these D-A assemblies into polymeric structures produces innovative materials with applications in different fields, including the photocatalytic degradation of organic pollutants in aqueous media.
We believe that the approaches outlined in this feature article concerning the precise modification of frontier molecular orbital energies and topologies in D-A assemblies based on oligothiophene–naphthalimide semiconductors can pave the way in the near future for the design and synthesis of more efficient materials for (opto)electronics and photocatalysis.