1000/1000
Hot
Most Recent

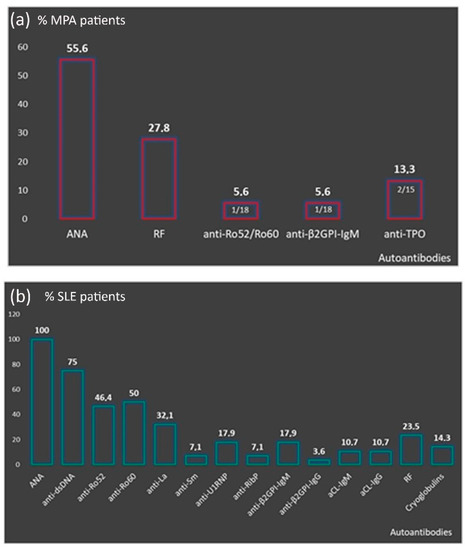
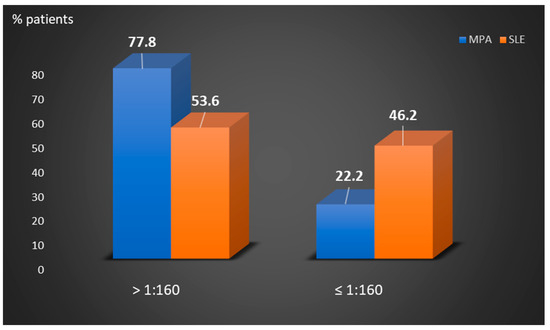
Perinuclear anti-neutrophilic cytoplasmic antibodies (P-ANCA) recognize heterogeneous antigens, including myeloperoxidase (MPO), lactoferrin, elastase, cathepsin-G and bactericidal/permeability-increasing protein. Although P-ANCA have diagnostic utility in vasculitides, they may also be found in patients with various other systemic autoimmune rheumatic diseases (SARDs). Nevertheless, the clinical significance and the targets recognized by P-ANCA in such patients remain unclear. For this purpose, herein we investigated the occurrence of ANCA-related antigenic specificities in 82 P-ANCA-positive sera by multiplex ELISA, as well as their association with other autoantibodies. The P-ANCA-positive sera corresponded to patients with vasculitides (n = 24), systemic lupus erythematosus (n = 28), antiphospholipid syndrome (n = 5), Sjögren’s syndrome (n = 7), rheumatoid arthritis (n = 3), systemic scleroderma (n = 1), sarcoidosis (n = 1) and Hashimoto′s thyroiditis (n = 13). In most P-ANCA-positive patients studied (51/82, 62.3%), these autoantibodies occurred in high titers (>1:160). The analysis of P-ANCA-positive sera revealed reactivity to MPO in only 50% of patients with vasculitides, whereas it was infrequent in the other disease groups studied. Reactivity to other P-ANCA-related autoantigens was also rarely detected. Our findings support that high P-ANCA titers occur in SARD. The P-ANCA-positive staining pattern is associated with MPO specificity in vasculitides, while in other autoimmune diseases, it mostly involves unknown autoantigens.
| Patient Groups | P-ANCA Serum Titers (No Positive) | ||||||
|---|---|---|---|---|---|---|---|
| ≥1:640 | 1:320 | 1:160 | 1:80 | 1:40 | 1:20 | ||
| Vasculitides | MPA (n = 18) | 9 | 5 | 1 | 1 | 1 | 1 |
| BD (n = 2) | 1 | 1 | 0 | 0 | 0 | 0 | |
| Aortitis (n = 1) | 1 | 0 | 0 | 0 | 0 | 0 | |
| HSP (n = 2) | 0 | 0 | 1 | 1 | 0 | 0 | |
| CV (n = 1) | 0 | 1 | 0 | 0 | 0 | 0 | |
| SLE (n = 28) | 9 | 6 | 3 | 7 | 23 | 0 | |
| APS (n = 5) | 1 | 1 | 1 | 1 | 0 | 1 | |
| SS (n = 7) | 5 | 0 | 0 | 0 | 0 | 2 | |
| RA (n = 3) | 2 | 0 | 0 | 1 | 0 | 0 | |
| SSCL (n = 1) | 0 | 1 | 0 | 0 | 0 | 0 | |
| Sarcoidosis | (n = 1) | 0 | 0 | 1 | 0 | 0 | 0 |
| Hashimoto | (n = 13) | 2 | 1 | 4 | 2 | 1 | 3 |
| Autoimmune Diseases | Antigens Recognized by P-ANCA Positive Sera (No Positive) | ||||||
|---|---|---|---|---|---|---|---|
| MPO | Elastase | Cathepsin G | BPI | Lactoferrin | MPO/Lactoferrin | ||
| Vasculitides | MPA (n = 18) | 11 | 0 | 0 | 0 | 0 | 0 |
| BD (n = 2) | 1 | 0 | 0 | 0 | 0 | 0 | |
| Aortitis (n = 1) | 0 | 0 | 0 | 0 | 0 | 0 | |
| HSP (n = 2) | 0 | 0 | 0 | 0 | 0 | 0 | |
| CV (n = 1) | 0 | 0 | 0 | 0 | 1/1 (100) | 0 | |
| SLE (n = 28) | 1 | 0 | 0 | 0 | 1/28 (3.6) | 1/28 (3.6) | |
| APS (n = 5) | 1 | 0 | 0 | 0 | 0 | 0 | |
| SS (n = 7) | 0 | 1 | 0 | 0 | 0 | 0 | |
| RA (n = 3) | 1 | 0 | 0 | 0 | 0 | 0 | |
| SSCL (n = 2) | 1 | 0 | 0 | 0 | 0 | 0 | |
| Sarcoidosis (n = 1) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hashimoto (n = 13) | 0 | 0 | 0 | 0 | 0 | 0 | |