1000/1000
Hot
Most Recent

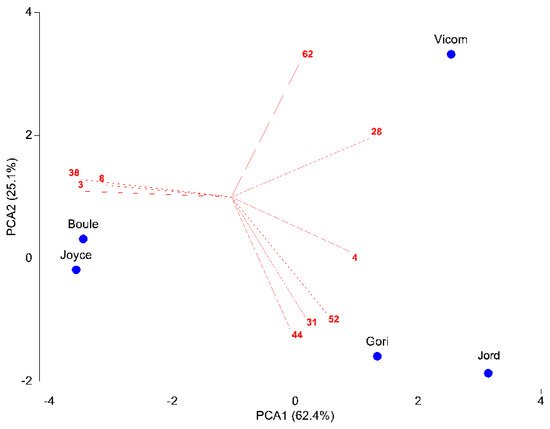
The present study reported the investigation of the chemical profile and the extraction yield of the essential oils (EOs) obtained from the dried aerial parts of four cultivars of Salvia rosmarinus (‘Boule’; ‘Vicomte de Noailles’; ‘Gorizia’; ‘Joyce de Baggio’) and the species S. jordanii, together with their antibacterial and antifungal activities. The phytochemical investigation evidenced a predominance of oxygenated monoterpenes in all the samples (57.5–77.1%), except in ‘Boule’, in which the hydrocarbon form prevailed (50.2%).
| Peak | Compounds | l.r.i. | Class. | Relative Abundances (%) ±SD | ||||
|---|---|---|---|---|---|---|---|---|
| Boule | Gori | Joyce | Vicom | Jord | ||||
| 1 | tricyclene | 922 | mh | - | 0.1 ± 0.00 | - | - | 0.1 ± 0.01 |
| 2 | α-thujene | 926 | mh | - | 0.2 ± 0.02 | 0.1 ± 0.00 | 0.2 ± 0.02 | - |
| 3 | α-pinene | 933 | mh | 37.3 ± 3.09 | 6.4 ± 0.10 | 25.6 ± 0.02 | 4.1 ± 0.04 | 3.1 ± 0.17 |
| 4 | camphene | 948 | mh | 2.9 ± 0.09 | 4.3 ± 0.04 | 2.0 ± 0.09 | 3.3 ± 0.10 | 3.9 ± 0.06 |
| 5 | thuja-2,4(10)-diene | 954 | mh | 0.3 ± 0.02 | - | 0.4 ± 0.07 | - | - |
| 6 | β-pinene | 977 | mh | 0.5 ± 0.04 | 4.2 ± 0.40 | 1.8 ± 0.07 | 1.6 ± 0.03 | 0.9 ± 0.01 |
| 7 | 3-octanone | 985 | nt | - | 1.0 ± 0.17 | - | - | - |
| 8 | myrcene | 991 | mh | 2.0 ± 0.16 | 0.6 ± 0.01 | 0.9 ± 0.09 | 0.4 ± 0.03 | 0.2 ± 0.02 |
| 9 | α-phellandrene | 1006 | mh | - | 1.2 ± 0.05 | 0.2 ± 0.00 | - | - |
| 10 | δ-3-carene | 1011 | mh | - | 0.4 ± 0.01 | - | - | - |
| 11 | α-terpinene | 1017 | mh | 0.2 ± 0.01 | 0.5 ± 0.02 | 0.5 ± 0.01 | 0.3 ± 0.01 | 0.7 ± 0.01 |
| 12 | p-cymene | 1025 | mh | 2.8 ± 0.22 | 1.0 ± 0.23 | 0.8 ± 0.08 | 1.8 ± 0.09 | 1.6 ± 0.06 |
| 13 | limonene | 1029 | mh | 3.3 ± 0.11 | 3.6 ± 0.05 | 2.1 ± 0.12 | 1.7 ± 0.14 | 1.7 ± 0.03 |
| 14 | 1,8-cineole | 1031 | om | 11.4 ± 0.22 | 20.5 ± 0.73 | 23.9 ± 0.42 | 20.0 ± 0.64 | 11.5 ± 0.11 |
| 15 | (Z)-β-ocimene | 1036 | om | - | - | - | - | 1.2 ± 0.10 |
| 16 | γ-terpinene | 1058 | mh | 0.4 ± 0.03 | 1.0 ± 0.10 | 0.9 ± 0.02 | 0.6 ± 0.02 | 0.7 ± 0.02 |
| 17 | cis-sabinene hydrate | 1066 | om | - | 0.3 ± 0.02 | - | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 18 | terpinolene | 1089 | mh | 0.5 ± 0.02 | 0.6 ± 0.01 | 0.7 ± 0.03 | 0.4 ± 0.00 | 0.2 ± 0.01 |
| 19 | trans-sabinene hydrate | 1098 | om | - | 0.1 ± 0.02 | - | - | - |
| 20 | linalool | 1101 | om | 1.5 ± 0.01 | 0.4 ± 0.04 | 2.1 ± 0.14 | 0.2 ± 0.01 | - |
| 21 | filifolone | 1108 | om | 0.2 ± 0.00 | - | - | - | - |
| 22 | fenchol | 1114 | om | 0.1 ± 0.01 | - | - | - | - |
| 23 | cis-p-menth-2-en-1-ol | 1122 | om | - | - | 0.2 ± 0.03 | - | - |
| 24 | α-campholenal | 1125 | om | - | - | - | 0.2 ± 0.01 | - |
| 25 | chrysanthenone | 1126 | om | 0.8 ± 0.05 | 0.2 ± 0.06 | 0.2 ± 0.01 | - | - |
| 26 | trans-pinocarveol | 1139 | om | 0.2 ± 0.02 | 0.1 ± 0.02 | - | - | - |
| 27 | cis-verbenol | 1142 | om | - | 0.1 ± 0.03 | 0.1 ± 0.03 | - | - |
| 28 | camphor | 1145 | om | 7.7 ± 0.22 | 16.9 ± 1.35 | 3.3 ± 0.50 | 42.2 ± 0.52 | 33.4 ± 0.38 |
| 29 | trans-pinocampone | 1160 | om | 0.3 ± 0.01 | - | 0.2 ± 0.01 | 0.2 ± 0.02 | - |
| 30 | pinocarvone | 1163 | om | 0.2 ± 0.00 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.3 ± 0.07 | - |
| 31 | borneol | 1165 | om | 2.5 ± 0.11 | 6.5 ± 0.00 | 3.7 ± 0.26 | 0.6 ± 0.13 | 14.6 ± 0.01 |
| 32 | isopinocampheol | 1173 | om | 0.4 ± 0.04 | - | - | - | - |
| 33 | cis-pinocamphone | 1174 | om | - | 0.8 ± 0.02 | 0.8 ± 0.01 | 0.5 ± 0.00 | - |
| 34 | 4-terpineol | 1177 | om | 1.4 ± 0.08 | 0.9 ± 0.08 | 1.1 ± 0.01 | 1.2 ± 0.02 | 2.8 ± 0.03 |
| 35 | p-cymen-8-ol | 1185 | om | 0.1 ± 0.01 | - | - | 0.2 ± 0.01 | - |
| 36 | α-terpineol | 1191 | om | 2.6 ± 0.19 | 2.0 ± 0.24 | 2.4 ± 0.09 | 2.8 ± 0.07 | 2.9 ± 0.06 |
| 37 | myrtenol | 1195 | om | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.4 ± 0.14 | 0.2 ± 0.03 | - |
| 38 | verbenone | 1210 | om | 12.8 ± 2.67 | 1.9 ± 0.15 | 14.9 ± 0.27 | 2.7 ± 0.03 | 0.6 ± 0.02 |
| 39 | trans-carveol | 1219 | om | 0.1 ± 0.06 | - | - | 0.2 ± 0.04 | - |
| 40 | carvone | 1244 | om | - | - | - | 0.1 ± 0.03 | - |
| 41 | geraniol | 1254 | om | 0.5 ± 0.08 | - | 3.9 ± 0.15 | - | - |
| 42 | trans-ascaridol glycol | 1268 | om | - | 0.4 ± 0.10 | - | - | - |
| 43 | geranial | 1271 | om | - | - | 0.3 ± 0.02 | - | - |
| 44 | bornyl acetate | 1286 | om | 3.9 ± 0.45 | 6.5 ± 0.50 | 2.6 ± 0.17 | 0.2 ± 0.01 | 10.8 ± 0.06 |
| 45 | myrtenyl acetate | 1326 | om | - | - | 0.1 ± 0.00 | - | - |
| 46 | eugenol | 1357 | pp | - | - | - | - | 0.4 ± 0.02 |
| 47 | α-copaene | 1376 | sh | - | 0.3 ± 0.05 | - | - | - |
| 48 | geranyl acetate | 1385 | om | - | - | 0.5 ± 0.03 | - | - |
| 49 | (Z)-jasmone | 1397 | nt | 0.4 ± 0.07 | - | - | - | - |
| 50 | methyl eugenol | 1407 | pp | - | - | 0.3 ± 0.02 | - | - |
| 51 | β-caryophyllene | 1419 | sh | 0.2 ± 0.03 | 6.7 ± 1.51 | 1.1 ± 0.15 | 0.6 ± 0.06 | 3.1 ± 0.08 |
| 52 | α-humulene | 1453 | sh | - | 1.9 ± 0.39 | 0.3 ± 0.04 | - | 3.2 ± 0.10 |
| 53 | γ-muurolene | 1477 | sh | - | 0.4 ± 0.08 | - | - | - |
| 54 | bicyclogermacrene | 1496 | sh | - | 0.3 ± 0.05 | - | - | - |
| 55 | trans-γ-cadinene | 1514 | sh | - | 0.4 ± 0.07 | - | - | - |
| 56 | δ-cadinene | 1524 | sh | - | 0.9 ± 0.20 | - | - | - |
| 57 | caryophyllene oxide | 1582 | os | 0.4 ± 0.09 | 4.3 ± 0.16 | 0.6 ± 0.10 | 1.5 ± 0.24 | 0.8 ± 0.02 |
| 58 | humulene oxide II | 1608 | os | 0.3 ± 0.07 | 0.5 ± 0.08 | - | - | 0.6 ± 0.03 |
| 59 | caryophylla-4(14),8(15)-dien-5-ol (unidentified isomer) | 1633 | os | - | 0.2 ± 0.06 | - | 0.3 ± 0.04 | - |
| 60 | T-cadinol | 1641 | os | - | 0.3 ± 0.03 | - | 0.2 ± 0.03 | - |
| 61 | α-bisabolol oxide B | 1655 | os | - | - | - | 0.8 ± 0.06 | - |
| 62 | 14-hydroxy-9-epi-(E)-caryophyllene | 1670 | os | - | - | - | 7.1 ± 1.76 | 0.2 ± 0.01 |
| 63 | α-bisabolol | 1685 | os | - | - | - | - | 0.4 ± 0.01 |
| 64 | trans-ferruginol | 2325 | od | 0.2 ± 0.04 | 0.2 ± 0.01 | - | - | - |
| Total identified (%) | 98.6 ± 0.06 | 98.7 ± 0.31 | 99.1 ± 0.16 | 96.6 ± 0.16 | 100 ± 0.03 | |||
| Boule | Gori | Joyce | Vicom | Jord | ||||
| Monoterpene hydrocarbons (mh) | 50.2 ± 3.77 A | 24.0 ± 0.54 C | 36.0 ± 0.19 B | 14.3 ± 0.46 D | 14.2 ± 0.49 D | |||
| Oxygenated monoterpenes (om) | 46.9 ± 3.42 C | 57.5 ± 2.08 B | 60.9 ± 0.04 B | 71.9 ± 1.55 A | 77.1 ± 0.25 A | |||
| Sesquiterpene hydrocarbons (sh) | 0.2 ± 0.03 C | 10.7 ± 2.35 A | 1.3 ± 0.19 C | 0.6 ± 0.06 C | 6.3 ± 0.18 B | |||
| Oxygenated sesquiterpenes (os) | 0.7 ± 0.16 C | 5.3 ± 0.33 B | 0.6 ± 0.10 C | 9.8 ± 2.12 A | 2.0 ± 0.07 C | |||
| Oxygenates diterpenes (od) | 0.2 ± 0.04 A | 0.2 ± 0.01 A | - B | - B | - B | |||
| Phenylpropanoids (pp) | - | - | 0.3 ± 0.02 | - | 0.4 ± 0.02 | |||
| Other non-terpene derivates (nt) | 0.4 ± 0.07 B | 1.0 ± 0.17 A | - C | - C | - C | |||
| EO Extraction yield (%w/w) | 0.57 ± 0.02 C | 1.17 ± 0.16 B | 0.76 ± 0.04 C | 2.25 ± 0.15 A | 0.71 ± 0.04 C | |||
| Antibiotics | ||||||
|---|---|---|---|---|---|---|
| STRAINS | Tetracycline (30 μg/disc) |
Ceftazidime (30 μg/disc) |
Rifampicin (30 μg/disc) |
Cephalexin (30 μg/disc) |
Cefotaxime (30 μg/disc) |
Chloramphenicol (30 μg/disc) |
| S. ser. Typhimurium (S176) | 18 (S) | 19 (S) | 15 (R) | 21 (S) | 25 (S) | 21(S) |
| Y. enterocolitica (YU3) | 22 (S) | 27 (S) | 17 (I) | 0 (R) | 32 (S) | 22 (S) |
| L. monocytogenes (L1) | 26 (S) | 0 (R) | 28 (S) | 21 (S) | 10 (R) | 22 (S) |
| E. durans (EU157) | 24 (S) | 0 (R) | 33 (S) | 14 (R) | 0 (R) | 19 (S) |
| E. faecium (EU107) | 7 (R) | 0 (R) | 30 (S) | 0 (R) | 0 (R) | 18 (S) |
| E. faecalis (EU37) | 10 (R) | 0 (R) | 15 (R) | 13 (R) | 18 (I) | 19 (S) |