Toll-like receptors (TLRs) represent a family of pattern recognition receptors that recognize certain pathogen-associated molecular patterns and damage-associated molecular patterns. TLRs are highly interesting to researchers including immunologists because of the involvement in various diseases including cancers, allergies, autoimmunity, infections, and inflammation. After ligand engagement, TLRs trigger multiple signaling pathways involving nuclear factor-κB (NF-κB), interferon-regulatory factors (IRFs), and mitogen-activated protein kinases (MAPKs) for the production of various cytokines that play an important role in diseases like cancer. TLR activation in immune as well as cancer cells may prevent the formation and growth of a tumor. Nonetheless, under certain conditions, either hyperactivation or hypoactivation of TLRs supports the survival and metastasis of a tumor. Therefore, the design of TLR-targeting agonists as well as antagonists is a promising immunotherapeutic approach to cancer.
1. Introduction
Toll-like receptors (TLRs) belong to the family of pathogen recognition receptors (PRRs) in the innate immune system and recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) related to foreign invading pathogens and host cells, respectively. To date, 13 TLRs have been reported in mice and 10 in humans, which are expressed on various immune cells (dendritic cells (DCs), macrophages, T-cell subsets, and B cells) and nonimmune cells (epithelial cells and fibroblasts) in humans. Based on their subcellular localization in humans, TLRs can be subdivided into two main groups: TLRs 1, 2, 4, 5, and 6 are present on the plasma membrane of cells; in contrast, TLRs 3, 7, 8, and 9 are localized in the endosomal membrane
[1]. Each type of TLR recognizes its specific ligand(s) and activates the associated signaling pathway in either a myeloid differentiation primary response protein 88 (MyD88)- or TIR domain–containing adaptor inducing IFNβ (TRIF)-dependent manner. The activation of signaling leads to the secretion of various cytokines, which help the host body to combat various invaders. Furthermore, TLRs are responsible for maturation of DCs, which link innate and adaptive immune responses
[2].
TLRs belong to the class of integral membrane type I glycoproteins, which have three major domains: the extracellular domain with different numbers of leucine-rich repeat (LRR) motifs, the transmembrane domain, and the cytoplasmic domain (similar to that of interleukin-1 receptor; IL-1R), which is known as the Toll/IL-1R (TIR) domain
[3]. The extracellular domain has 19–25 tandem LRR motifs (20–26 in case of mammals), and each one of them contains 24–29 amino acid residues, including conserved residues (XØXXØXXXXFXXLX; Ø = hydrophobic residue) as well as motif XLXXLXLXX. Each LRR motif forms an α-helix and β-strand separated by a loop, which eventually forms a horseshoe structure after ligand binding on its inner concave surface in most of TLRs except TLR3, where the ligand binds to the outer convex surface
[4].
Upon ligand recognition, TLRs undergo conformational changes to form homo- or heterodimers, which induce their TIR domain to interact with the TIR domain of intracellular adaptor molecules including MyD88, TIR domain–containing adaptor protein (TIRAP, i.e., Mal), TRIF, i.e., TICAM1, and TRIF-related adaptor molecule (TRAM, i.e., TICAM2)
[5]. The details of this signaling pathway are described elsewhere
[6]. Briefly, relevant adaptor molecules recruit various members of the interleukin 1 receptor–associated kinase (IRAK) family, in turn activating tumor necrosis factor receptor–associated factor 6 (TRAF6), whose ubiquitination switches on transforming growth factor β (TGF-β)-activated protein kinase 1 (TAK1). The latter causes the inhibitor of κB kinase (IKK) complex to stimulate the activity of a transcription factor (nuclear factor kappa-light-chain enhancer of activated B cells; NF-κB) and various mitogen-activated protein kinases (MAPKs) to trigger c-Jun N-terminal kinase (JNK), protein 38 (p38), and extracellular signal–regulated kinase (ERK), which turns on a transcription factor called activated protein 1 (AP-1)
[7]. TLR3 and TLR4 employ TRIF/TRAM adaptor molecules to launch the activity of interferon response factor (IRF) 3, whereas TLR7, TLR8, and TLR9 activate IRF7. These transcription factors move into the nucleus and initiate the expression of various target genes including inflammatory cytokines, chemokines, and type I interferons (IFNs).
Cancer cells divide uncontrollably unlike the ~200 types of somatic cells in our body, which can double 50–60 times before entering the state of senescence
[8]. The cancer hallmarks include tumor metastasis and angiogenesis and tumor cell survival and proliferation
[9]. The conversion to malignancy and cancer progression involve downregulation of tumor suppressor genes and upregulation of proto-oncogenes and associated signaling pathways
[9][10]. Moreover, several cellular and molecular mechanisms help tumors escape the body’s own natural immune response
[11][12]. The importance of immune regulation for cancer progression can be explained by the presence of increased amounts of immunosuppressive factors and cells and by scarcity of immune-system–activating signals in a tumor microenvironment. Under this scenario, it is worthwhile to activate immune cells through the receptors on their surfaces; one of these receptors is a TLR. Its activation is a double-edged sword, i.e., the exact pro- or antitumor effect depends upon the type of TLR, the cell type expressing it, and the downstream signaling cascade in such cells. For anticancer therapies, TLR agonists are being explored as vaccine adjuvants for the stimulation of immune cells and promotion of inflammation. TLR activity also upregulates the expression of such costimulatory molecules as CD40, CD80 (B7.1), and CD86 (B7.2) and cytokines like IL-12, which stimulates other immune cells like T lymphocytes
[2][13]. In contrast, TLR expression and triggering on other cells including cancer cells can lead to tumor growth
[14]. Active TLR signaling regulates the expression of various genes involved in tumor progression ().
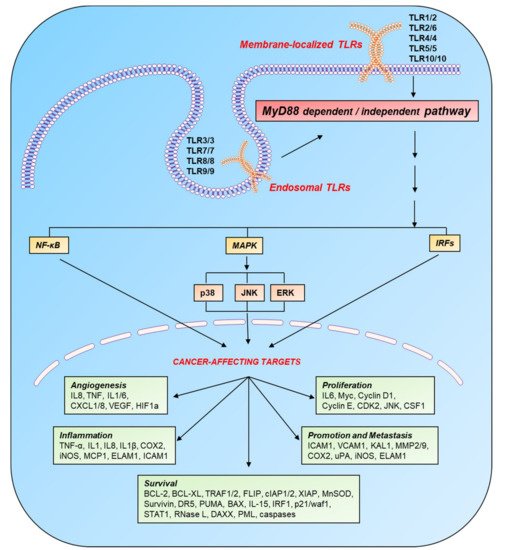
Figure 1. Induction of cancer-affecting genes by TLR signaling. TLRs are localized on the cell surface and in the endosomal compartment and become active after recognizing their respective PAMPS and DAMPs. On the basis of intracellular adaptor molecules, TLR pathways are categorized into two main cascades: MyD88-dependent and MyD88-independent. These pathways switch on various transcription factors: p50/p65, AP-1, and IRFs through NF-κB, MAPK, and IFN pathways, respectively. These transcription factors target various genes (involved in the processes of inflammation, angiogenesis, cell survival, proliferation, and metastasis), which directly or indirectly affect the progression of cancer. Legend: AP-1, activated protein 1; BAX, BCL2-associated X; BCL, B-cell lymphoma protein; CDK, cyclin-dependent kinase; cIAP, cellular inhibitor of apoptosis protein; COX, cyclooxygenase; CSF, colony-stimulating factor; CXCL, chemokine (C-X-C motif) ligand; DAXX, death domain–associated protein; DR, death receptor; ELAM, endothelial-leukocyte adhesion molecule; ERK, extracellular signal–regulated kinase; FLIP, FLICE-like inhibitory protein; HIF, hypoxia-inducible factor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; iNOS, inducible NO synthase; IRF, interferon response factor; JNK, c-Jun N-terminal kinase; KAL, Kallmann syndrome gene; MAPK, mitogen-activated protein kinase; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; MnSOD, manganese superoxide dismutase; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor κB; p38, protein 38; PML, promyelocytic leukemia protein; PUMA, p53-upregulated modulator of apoptosis; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; TNF-α, tumor necrosis factor α; TRAF, tumor necrosis factor receptor (TNF-R)-associated factor; uPA, urokinase-type plasminogen activator; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; WAF, wild-type activating fragment; XIAP, x-linked inhibitor of apoptosis protein.
2. TLR Signaling in Immune and Cancer Cells
The pro- or antitumor effect of TLR signaling is determined by the specific TLR being stimulated, the cell type with activated signaling, and a downstream signaling cascade in the activated cells. Various TLR agonists promote inflammation by activating immune cells and are currently tested in clinical trials of anticancer therapies as vaccine adjuvants. TLRs are expressed on many cell types in humans but are mainly detectable on DCs, monocytes, and mature macrophages
[15]. Ligand engagement by TLRs on these cells causes overexpression of multiple membrane-bound costimulatory molecules like CD40, B7.1 (CD80), and B7.2 (CD86) along with cytokines needed for proper T-cell activation such as IL-12
[2][13]. Aside from TLR activation on antigen-presenting cells (APCs), TLR activation in other cells also plays a substantial part in tumor growth. For instance, tumor cells and T cells express various TLRs that are activated after recognition of their associated ligands
[14][16]. Proper communication between immune and cancer cells via cytokines or costimulatory molecules exerts an antitumor effect ().
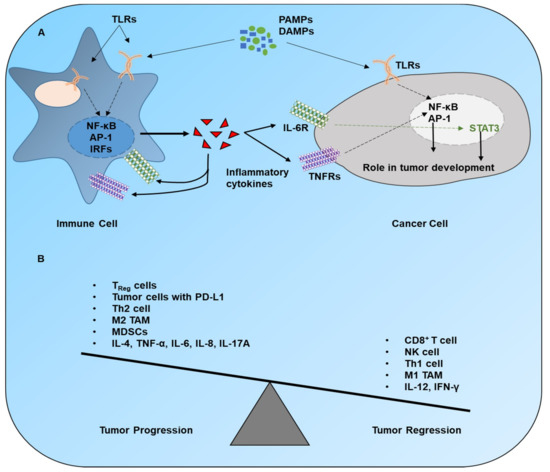
Figure 2. Immune regulation of cancer progression. (A) The stimulation of TLRs and other PRRs in immune cells launches downstream signaling pathways, which cause a release of various cytokines. These cytokines interact with their receptors on immune cells and cancer cells to trigger associated signaling pathways. The product(s) of these cascades plays a substantial role in the progression of cancer. (B) The overall outcome of cancer in a tumor microenvironment depends upon the ratio of protumor to antitumor signals. Legend: CD, cluster of differentiation; DAMPs, damage-associated molecular patterns; IFN, interferon; IL, interleukin; MDSCs, myeloid-derived suppressor cells; NK, natural killer; PAMPs, pathogen-associated molecular patterns; PD-L1, programmed-death ligand 1; TAM, tumor-associated macrophage; Th2, T helper type 2; TNF-α, tumor necrosis factor α; Treg, T regulatory.
Treatment of mouse models with TLR agonists lessens the growth of tumors and even destroys established tumors in some cases during a combinatorial therapy with other agents such as monoclonal antibodies, chemotherapy drugs, and antigenic vaccines, e.g., plasmid DNA, peptides, or proteins
[17][18][19][20][21][22].
The expression profile of TLRs on T cells depends upon the T-cell subset in question as well as their activation status. Lower levels of TLR mRNA and protein are detected in naïve T cells and increase dramatically upon their stimulation via TCR or by such compounds as PMA or ionomycin
[23]. Similarly, stand-alone activation of TLRs has a smaller effect on resting or naïve T cells, but TLRs exert costimulatory effects on T cells during the stimulation of TCR
[24][25]. Nonetheless, TLR expression is transient and diminishes with the passage of time
[23][24][26]. Notably, some TLRs are expressed more weakly in human and murine memory T cells than in activated T cells, but this level is enough for them to respond in the absence of triggering by TCR
[27][28].
2.1. TLR Signaling in DC Subsets
In the immune system, DCs are considered most efficient professional APCs. Immature DCs undergo infection- or inflammation-mediated activation and differentiation into mature DCs that activate the cells of adaptive immunity such as B and T lymphocytes
[29]. The maturation of DCs involves a series of steps such as reduced changes in the sets of receptors of endocytosis and phagocytosis; overexpression of costimulatory molecules including CD40, CD58, and CD86; morphological changes; and reshuffling of lysosomal and MHC compartments. The DC population is a heterogeneous collection of various subtypes that differ in their function, phenotype, and localization. Two main populations are found in peripheral human blood, which include CD11c-negative plasmacytoid DCs (pDCs), CD11c-positive myeloid DCs (mDCs), monocyte-derived DCs (moDCs), and CD34
+ cell–derived DCs
[30]. These subsets express distinctive PRRs, which allow them to perform specialized functions by detecting different pathogenic stimuli. DCs take up pathogens and present pathogen-derived processed peptides to T cells by means of major histocompatibility complex (MHC) molecules. The activation status of DCs determines the overall outcome of an immune response. For example, resting DCs or those receiving inhibitory signals (such as corticosteroids or IL-10) promote immune tolerance by inducing upregulation of regulatory T (T
reg) cells or elimination of effector T cells; however, mature DCs evoke immunity. T cells are activated after recognition of the peptides processed and expressed by DCs (signal 1) and stimulation by cytokines (signal 2) and costimulatory molecules (signal 3).
TLR expression on DCs depends upon subtypes, species, and maturation stage. All three subpopulations of mDCs (i.e., CD1c
− mDCs, CD16
− mDCs, and BDCA3
− mDCs) express TLRs 1–10 except TLR3 (absent only in CD16
− mDCs) at the RNA level. Both CD1c
− mDCs and CD16
− mDCs respond strongly to agonists of all TLRs except agonists of TLR9 (CpG oligodeoxynucleotides)
[31]. Of note, Cd16
− mDCs respond to the TLR3 ligand poly(I:C) despite the absence of
TLR3 RNA, in a TLR3-independent manner, possibly because of either cytosolic RNA sensors
[32] or minor contamination with endotoxins. The CD1c
− mDCs and moDCs both express similar endosomal (TLR3 and TLR8) and extracellular TLRs (TLRs 1, 2, 4, 5, and 6), which allow them to produce inflammatory cytokines after stimulation by the respective ligands
[33][34]. Nonetheless, both subsets do not express TLR9, whereas TLR10 is expressed only by mDCs
[34][35]. On the other hand, pDCs express TLR1 weakly, which is nonresponsive to the ligand of TLR1/2 owing to the absence of TLR2
[34]. Despite the absence of TLR3 and TLR8 expression, pDCs respond to viral pathogens, probably via TLR7, which binds to the same ligand and shares the signaling pathway. In addition, pDCs express TLR10 whose binding partner and function are still unknown
[36][37].
The distinct profile of TLRs in various subsets indicates that mDCs mainly respond to fungal and bacterial antigens, whereas pDCs mainly respond to viral pathogens. These subsets can be used in DC vaccination therapy for an antitumor and Th1 response
[35][38][39]. Human pDCs can infiltrate various tumors such as ovarian cancer
[40], head and neck cancer
[41], and breast cancer
[42]. The differentiation and maturation of infiltrating pDCs are prevented by the suppressive environment created by soluble factors secreted from a tumor
[43][44][45]. Despite infiltration, pDCs cannot become activated after sensing DNA at the site of infiltration. These phenomena lead to the induction of T
reg cells and a poor prognosis
[42][46]. Other studies have revealed that the recruitment of pDCs and the production of type I IFNs can be enhanced by a TLR7 agonist (imiquimod), which causes tumor regression by creating an inflammatory environment
[47][48]. Similarly, an antitumor response has been observed in melanoma skin metastases and basal cell carcinoma after pDC activation by intratumoral injection of a TLR9 agonist, a CpG oligodeoxynucleotide
[49]. This agonist will activate only pDCs because of the absence of TLR9 on mDCs. By contrast, a TLR7/8 ligand (R848) can stimulate both pDCs and mDCs, and this approach will be more effective in eliciting an antitumor response at the tumor site.
Recent studies showed a cooperative and synergistic association between mDCs and pDCs. Along with direct induction of a CD8
+ T-cell response specific to a tumor antigen, pDCs stimulate the tumor antigen-presenting ability of mDCs toward T cells
[50]. Moreover, both of these human DC subsets stimulate each other when any of them is activated in vitro by its respective TLR ligand
[51]. In clinical settings, the research in this field suggests that DC vaccination with both mDCs and pDCs is more effective than vaccination with moDCs alone in terms of an antitumor response
[51].
2.2. TLR Signaling in T-Cell Subsets
2.2.1. TLR1/2 and TLR2/6
The functional engagement of TLR1/2 on CD8
+ cells enhances the production of TNF-α
[52][53], IFNγ
[54], and IL-2
[52] along with cytolytic molecules like perforin and granzyme B
[55]. The importance of T-cell–mediated TLR signaling is emphasized by reversed or delayed tumor growth of B16 melanoma in tumor-bearing MyD88 knockout mice that received adoptive transfer of TCR-transgenic CD8
+ pmel T cells, after peritumoral injections of Pam3CSK4 (a synthetic TLR2 agonist)
[56]. In vitro and in vivo models have confirmed that a bacterial lipoprotein can kill tumor cells too by recruiting CD8
+ cells
[57][58].
Furthermore, the T-cell response is directly modulated by some DAMPs such as heat shock proteins. For example, CD45RA
+ naive and CD45RO
+ memory T cells show reduced chemotaxis and increased β1-integrin–dependent adhesion after downregulation of two chemokine receptors, CCR7 and CXCR4, because of Hsp60-mediated stimulation of TLR2 present on the surface of these cells
[59]. The interactions between T cells and tumor cells or APCs are mediated by the presence of integrins on T cells, and these integrins strengthen the cell activation
[60] and are considered the markers distinguishing between effector and memory T-cell subsets
[61].
A durable and effective immune response is hindered by the tolerance and immune suppression caused by T
reg cells. The cytolytic activity of tumor-specific CD8
+ T cells is compromised by the production of TGF-β and IL-10. Moreover, the suppressive function of T
reg cells is reduced by the stimulation of their TLR2 because CD8
+ T cells proliferate when grown in coculture with T
reg cells treated with a bacterial lipoprotein
[54]. Likewise, in in vitro and in vivo studies on murine T
reg cells, stimulated-TLR2–mediated reversal of the suppression is reported to be mediated by IL-2 and TCR activation
[62][63][64][65].
Low-affinity tumor antigens cause insufficient stimulation of TCR signaling thereby posing another problem for the effective antitumor response of T cells
[66][67]. Some other studies indicate formation of memory cells owing to weak TCR signaling after TLR1/2 activation on CD8
+ cells
[27][56][68]. Increased PKC and PI3K signaling activities cause costimulation of TLR signaling
[69][70]. An increase in the expression of T-bet and its enhanced binding to the promoter regions of granzyme B, IFNγ, and perforin genes is observed in CD8
+ cells after TLR stimulation
[55]. The importance of TCR activation is proven by the experiments showing that melanoma tumor growth is reduced by the injection of TCR-transgenic CD8
+ pmel T cells and of the TLR2 ligand Pam3CSK4 into tumor-bearing MyD88 knockout mice; this phenomenon is not observed in the mice injected only with pmel T cells and MyD88 knockout pmel or TLR2 knockout pmel T cells
[56]. Other studies have highlighted possible usefulness of lipopeptides as vaccines to generate a broad-spectrum T-cell repertoire via the triggering of TLR signaling in T cells
[71]. Enhanced degranulation and IFNγ production have been observed in γδT cells after TLR activation through TCR
[72].
Increased levels of IFNγ and IL-2 are observed in CD4
+ and CD45RO
+ cells after TLR2 stimulation
[25][52]. Memory CD4
+ T cells can be directly stimulated by TLR2-agonistic lipoproteins of
Mycobacterium tuberculosis that cause enhanced proliferation and production of IFNγ and IL-2. Along with TLR2 stimulation, these cells require associated TCR signaling for the proper response
[73]. Moreover, TLR2 agonists increase the longevity of T cells through downregulation of proapoptotic proteins like Bim and upregulation of antiapoptotic proteins such as Bcl-xL and A1
[25][56].
2.2.2. TLR3
TLR3 is expressed by activated CD4
+ T cells and enhances NF-κB–dependent cell survival and proliferation upon stimulation with a specific ligand, poly(I:C). The enhanced survival of cells is attributed to increased expression of antiapoptotic protein Bcl-xL
[74]. It is reported that poly(I:C) can enhance the response and proliferation of CD8
+ T cells independently of CD4
+ T cells and APCs
[57]. Moreover, the formation of memory T cells is enhanced by TLR3 costimulation via TCR; this phenomenon is attributed to the prolonged T-cell survival due to TLR3 signaling. In T cells, the ability of TLR3 signaling to obviate CD4- or APC-mediated costimulation and formation of memory T cells is a helpful feature for the design of cancer vaccines because costimulatory signals are absent in a tumor microenvironment
[28].
Among CD8
+ T cells of humans, IFNγ production by PHA-activated memory or effector T cells rises after stimulation with poly(I:C) but fails to increase their lytic activity
[75]. Similarly, proliferation of mouse CD8
+ T cells and their secretion of IFNγ were augmented after preincubation with antigen-pulsed splenocytes with poly(I:C). Unlike untreated CD8
+ T cells, those treated with the TLR3 ligand showed a greater expansion potential upon adoptive transfer and displayed higher amounts of high-affinity CD25 (IL-2R α-chain) and activation marker CD69
[76]. The enhanced expression of CD69 and increased production of IFNγ have been observed in TLR3-stimulated freshly isolated γδT cells
[77]. In vitro, granzyme A–mediated and granzyme B–mediated cytolytic activities of an expanding γδT cell population are augmented after TCR stimulation by bromohydrin pyrophosphate and pretreatment with poly(I:C)
[78]. Along with IFN-γ, TLR3 activation in T-cells might also lead to the secretion of IFN-β which plays a main role in antiviral response
[79][80][81].
2.2.3. TLR4
The activation and greater proliferation of T cells under the influence of LPS are mediated by stimulation of the production of a cascade of proinflammatory cytokines by APCs
[82]. LPS-induced TLR4 stimulation is also observed in CD4
+ and CD8
+ T cells that can produce TNF-α, IFNγ, granzyme B, and perforin
[83][84]. On the contrary, the expression of TLR4 and CD14 has not been detected in murine CD8
+ T cells
[84]. Nonetheless, murine naïve CD4
+ T cells show increased survival and proliferation upon LPS treatment unlike nonresponsive naïve murine T cells
[85]. Thorough analysis has revealed that
TLR4 mRNA is expressed by the Th17 subset of murine CD4
+ T cells and that LPS stimulation increases the level of IL-17A and reduces that of IFNγ because of decreased activation of MAPK
[86][87]. The activation of these cells by TLR4 can lead to colitis by aggravating intestinal inflammation.
LPS-induced activation of TLR4 in CD4
+CD25
+ T
reg cells enhances immunosuppressive activity and proliferation unlike the engagement of TLR1/2
[88]. On the other hand, HMGB1-induced triggering of TLR4 on T
reg cells reduces IL-10 production and the expression of forkhead box p3 and CTLA4
[70]. Furthermore, switched-on TLR4 in T
reg cells mainly launches signaling in a TRIF-dependent manner, but in nonregulatory T cells, this process is mediated by MyD88 and p38 MAPK
[88]. These studies highlight the ligand- and cell-dependent response after TLR4 engagement.
2.2.4. TLR5
TLR5 binding by its ligand (bacterial flagellin)—similarly to other TLR agonists—leads to the production of IL-8, IL-10, and IFNγ but not IL-4. The costimulatory effect of flagellin on effector memory CD4
+CCR7
− cells is stronger than the effect on CCR7
+ central memory cells
[89]. The induction of IFNγ in the absence of IL-4 elicits a Th1 reaction that facilitates an efficient response of CD8
+ T cells. The increased proliferation and production of TNF-α, IFNγ, and granzyme B under the influence of flagellin have also been observed in CD8
+ T cells of human cord blood. This response is surprisingly stronger when it is implemented in combination with Pam3CSK4 (a TLR2 agonist)
[89]. These synergistic effects of various TLR ligands highlight their usefulness in a combinatorial therapy designed to evoke an in vivo antitumor response of T cells. The activation of TLR5 on human CD4
+CD25
+ T
reg cells enhances the expansion of this subset with augmented suppressive activity in contrast to the inhibitory effects of other TLR agonists on murine T
reg cells
[90]. Nevertheless, it is necessary to confirm these results in vivo and elucidate the production of T
reg cell–inhibiting cytokines by macrophages and DCs.
2.2.5. TLR7/8
Human T
reg cells express TLR8, but naïve CD4
+ T cells do not. A TLR9 ligand, CpG-A, induces the production of IFNα and IFNβ, which facilitate the proliferation of effector CD4
+ T cells by reversing the suppressive actions of T
reg cells. Furthermore, an enhanced antitumor activity with a loss of suppressive activity by T
reg cells is observed in a tumor mouse model upon their adoptive transfer after pretreatment with poly-G10 (a TLR8 ligand)
[91]. Additionally, this study indicates that the TLR9 expressed on T
reg cells can recognize CpG DNA molecules too
[91]. Similarly, TLR8 activation on human suppressor γδT cells by ssRNA40 or poly-G3 reverses their in vivo and in vitro suppressive effects on CD4
+ T cells
[92]. The activation of TLR7/8 in human CD4
+ T helper cells by its synthetic ligand such as resiquimod (R-848) increases the production of IL-10, IL-2, and IFNγ with enhanced proliferation independent from APCs
[89].
2.2.6. TLR9
The survival and antitumor response of CD4
+ T cells are increased by TLR9 stimulation
[63]. The increased in vitro survival of TLR9-activated murine T cells is explained by the initiation of NF-κB signaling and enhanced expression of antiapoptotic protein Bcl-XL
[74]. It is reported that costimulation of T cells by a TLR9 ligand enables them to overcome their reliance on PKC-ϕ signaling and reverses their anergy status by re-establishing in vitro survival and proliferation
[93]. Moreover, the activation of CD4
+ T cells by a TLR9 ligand makes them resistant to the suppressive effect of T
reg cells
[94]. Additionally, TLR9 engagement increases the number of CD4
+ and CD8
+ T cells by boosting the expression of IL-2 and of its associated receptor IL-12R, which takes place even in the absence of costimulatory molecules (such as CD28) in a tumor microenvironment
[95]. A reduction in radiation-induced apoptosis and increased DNA repair are observed in TLR9-activated CD4
+ T cells
[96].
2.3. TLR Signaling in the Cancer Cell
TLRs are mainly expressed by immune cells such as macrophages, DCs, and T-cell subsets. Recent studies uncovered the expression of TLRs in various tumor cells
[97][98][99][100][101]. For instance, the majority of colon cancer cells overexpress TLR2, TLR3, and TLR4
[102][103]. Similarly, ovarian cancer cells overexpress TLR2, TLR3, TLR4, and TLR5
[104][105].
Researchers are focusing on the expression and function of TLRs in various cancers. Enhanced invasiveness of human gastric cells and greater vascularization of gastric tissue after the activation of TLR2 enhance tumor growth by inducing the production of IL-8, PGE2, and COX-2
[106]. Higher mRNA copy numbers of
TLR3 are observed in the colon mucosa of polyposis patients; this parameter is linked to the stages of colorectal cancer
[107]. A reduction in the incidence, size, and number of neoplasms induced by chemicals has been observed in TLR4- and MyD88-deficient mice, thus underscoring a supportive role of TLR signaling in hepatocarcinogenesis
[58][108]. The progression of human breast cancer is strengthened by the production of immunosuppressive factors (e.g., NO, IL-6, IL-12, VEGF, and MMPs) after the engagement of TLR4 by its ligand
[109][110][111]. In mouse models of colon cancer, stimulation of TLR4 leads to the overexpression of an ICOS ligand (B7-H2) and programmed cell death ligand 1 (B7-H1) as well as downregulation of death receptor Fas; these changes prolong tumor survival
[112]. The activation of TLR5 in human gastric cancers causes production of IL-8 and TNF-α, which lead to the proliferation of tumor cells
[113]. The expression of TLR5 and TLR9 is significantly increased in late-stage cervical cancer but not observed in normal cervical squamous epithelial cells
[114]. TLR7/8 activity enhances tumor growth, survival, metastasis, and inflammation in lung cancer patients
[115]. TLR9 expression enhances angiogenesis, which is linked to lower survival rates of lung cancer patients
[116]. Moreover, time- and dose-dependent proliferation of prostate cancer cells is observed during TLR9-mediated expression of NF-κB and c-Myc
[117].
Although the incidence and progression of various tumors are promoted by TLRs, some TLRs may possess an antitumor function.
Mycobacterium Bacillus Calmette-Guérin (BCG) is enriched with peptidoglycans and unmethylated CG-containing DNA, which stimulates TLR2, TLR4, and TLR9. Treatment with BCG reduces motility and proliferation and increases the apoptosis of cells of urothelial carcinomas
[118]. Inhibition of proliferation and promotion of apoptosis of prostate cancer cells are observed after the activation of protein kinases by an agonist of TLR3, poly(I:C)
[119]. Apoptosis of human colon cancer cells has been observed after combinatorial treatment with poly(I:C) and either IFNα or 5-fluorouracil
[120]. Increased TLR3 expression in human melanoma cells after pretreatment with a type I IFN results in the inhibition of proliferation with subsequent death of these tumor cells
[121]. The evidence of antitumor activity of TLR4 is scarce; however, its triggering on lung epithelial cells has a protective effect against the formation of a lung tumor
[122].
TLR5 signaling in breast cancer downregulates cyclins B1, D1, and E2 thus inhibiting the proliferation of the tumor cells
[123]. Increased apoptosis and decreased proliferation of head and neck cancer cells are seen after treatment with a TLR5 agonist, flagellin
[124]. Cell cycle arrest and reduced proliferation of human glioma cells are reported after the launch of downstream NO and NF-κB pathways by a TLR9 agonist (CpG ODN 107) and irradiation
[125]. Moreover, TLR9 activity enhances apoptosis of neuroblastoma cells and inhibits the angiogenesis in renal cell carcinoma
[126].
In a word, cells of various cancer types express various TLRs. Among them, TLR3 and TLR5 have more promising antitumor effects unlike TLR4, -7, -8, and -9. Of note, activation of a particular TLR on one type of tumor cells has an antitumor impact but may play a protumor role in another tumor type(s). Therefore, it is important to choose an optimal TLR agonist for tumor cells in question in order to ensure an antitumor effect. This choice should be based on the expression profile of TLRs and their functional outcome for this type of cancer.
3. TLRs as Therapeutic Targets in Cancers
3.1. TLR Agonism for Cancer Prevention or Treatment
3.1.1. TLR2/TLR4
Despite their protumor activity, TLR2 and TLR4 have been studied as components of adjuvants for vaccination and tumor therapy. For example, BCG switches on TLR2 and TLR4 thereby exerting antitumor immunomodulatory effects
[127][128][129][130][131] especially in bladder cancer (FDA approval has already been obtained)
[132][133][134]. Similarly, a derivative of
Escherichia coli lipid A, OM-174 (CXR-526), engaging both TLR2 and TLR4, is being tested in a phase I trial against a solid tumor and phase I/II trials as a vaccine adjuvant for melanoma treatment. Stimuvax contains monophosphoryl lipid A, which stimulates TLR4; it is useful against the MUC1 tumor antigen but does not alleviate non–small cell lung carcinoma
[135].
3.1.2. TLR3
The TLR3 ligand poly(I:C) has shown antitumor effects in several mouse studies
[136][137], but hardly any data are available regarding humans. Several alternative ligands of TLR3 are being developed because of the rapid degradation of poly(I:C). For example, a poly(I:C) derivative (poly-ICLC; Hiltonol®) is stabilized by poly-lysine and is being evaluated in a phase II clinical trial against a solid tumor. Another derivative, rintatolimod (Ampligen
®), features a substitution of cytidine with uridine at a 1:12 ratio and is used in the treatment of fallopian tube, ovarian, and brain tumors in combination with some vaccines. The administration of TLR3 ligand (poly(I:C)) reduces orthotopic prostate cancer in transgenic mice (TRAMP C57B16 × FvB F1 Tg
+/−) as well as TRAMP tumors subcutaneously implanted in syngenic mice
[136]. Similarly, single administration of poly(I:C) into B16-F10-induced mouse model of metastatic lung cancer arrested tumor growth in association with greater influx of dendritic cells (DCs) which created cytotoxic immune environment
[137].
3.1.3. TLR5
A TLR5 agonist, flagellin, and TLR5-agonistic nanoparticles have shown significant antitumor effects in mice
[123][138][139]. Entolimod (CBLB502) derived from
Salmonella flagellin
[140] is a TLR5 agonist that is in phase I clinical trials against squamous cell head and neck cancer and solid tumors. The treatment of breast cancer cells with TLR5 agonist (flagellin) activated the intrinsic signaling pathway which led to the inhibition of anchorage-independent growth and cell-proliferation
[123]. Another study showed the contrasting result of flagellin administration into mice subcutaneously transplanted with weak immunogenic tumor or its variant stably expressing strong antigenic HER-2 oncoprotein. Administration of flagellin after 8-10 days of tumor implantation significantly reduced the growth of antigenic variant tumor but not that of weakly immunogenic. In contrast, flagellin administration with antigenic-tumor implantation accelerated its growth. These contrasting results are because of increased ratio of IFN-γ:IL-4 and decreased number of CD4
+CD25
+ T regulatory cells in first case and vice versa. The early combinatorial treatment of flagellin with CpG-containing oligodeoxynucleotides completely suppressed the tumor growth
[138].
3.1.4. TLR7/8
TLR7/8 are the most effective among all TLRs with respect to immunomodulatory anticancer effects. Hence, the only TLR agonist approved for cancer therapy is the one targeting TLR7/8, imiquimod. Both of these TLRs are activated simultaneously by the same ligand because of their common ability to recognize single-stranded RNA. The agonists of TLR7/8 have been classified into guanosine and adenosine analogs such as imiquimod and loxoribine, respectively. The ligands can be targeted specifically to TLR7 or TLR8 after modification of their RNA sequence
[141]. Among all such ligands, imiquimod is in clinical use: the FDA and European Medicines Agency have approved Aldara (5% imiquimod cream) for the treatment of basal cell carcinoma, and this substance has a 42–100% clearance rate
[142][143]. It has also found applications in the treatment of other local cutaneous tumors, including lentigo maligna, with a >85% success rate and significant clearance of melanoma
[144]. Unlike imiquimod, 852A (a TLR7 agonist) and VTX-2337 (a TLR8 agonist) can be administered systemically and are being tested in phase I/II clinical trials against various malignant tumors, e.g., ovarian, breast, cervical, endometrial, and head and neck cancers.
3.1.5. TLR9
Various TLR9 agonists based on CpG oligodeoxynucleotides are being tested in animal models of neuroblastoma, cervical carcinoma, and colon cancer
[145][146][147][148][149] and in some clinical trials
[150]. Despite good preclinical results, several trials have been disappointing: there were safety issues of IMO-2055 with platinum-based therapies in a phase II trial against recurrent and metastatic head and neck cancer, and a phase III trial of CPG7909 failed against non–small cell lung cancer
[151]. As a combinatorial therapy, CPG7909 (a TLR9 agonist), monophosphoryl lipid A (a TLR4 agonist), and MAGE-A3 (a melanoma antigen) are currently evaluated in phase III clinical studies.
3.2. TLR Antagonism for Cancer Treatment or Prevention
The protumor effects of TLRs in such organs as the liver, colon, and pancreas necessitate inhibition of TLR signaling at these sites for cancer treatment. Unfortunately, the results of studies on animal models cannot be translated into clinical trials so far. Promising antagonistic strategies are discussed below.
3.2.1. Manipulation of the Gut Microbiota
The microbiota of intestines is rich in bacterial TLR ligands, which substantially participate in the carcinogenesis of the colon, stomach, and liver. TLR-mediated tumor-promoting and inflammatory signals can be reduced by modulating the bacterial translocation and/or gut microbiota by means of antibiotics or probiotics
[152]. In murine models of azoxymethane (AOM)-induced colon cancer, the formation of aberrant cryptic foci is prevented by synbiotics but not by a pro- or prebiotic alone
[153][154]. Administration of probiotic VSL#3 in a rat model of liver carcinogenesis reduces the formation of a liver tumor
[155]. In a genetic murine model of colorectal cancer, the expression of protumorigenic IL-23 in tumor-associated macrophages is reduced by short-term treatment with an antibiotic, whereas the number and size of tumors decrease with long-term suppression of the gut microbiota
[156]. A reduction in the formation of colonic dysplasia is observed after sterilization of the gut in a colon cancer model
[157]. The tumor burden in rat and mouse models is drastically reduced by sterilization of the gut with oral antibiotics
[158][159]. The antibiotics are more effective if administered at later stages of hepatocarcinogenesis, indicating a possible role of the gut microbiota in cancer prevention, where early treatment is not possible. In murine hepatocarcinogenesis, a nonabsorbable well-tolerated antibiotic like rifaximin can decrease liver tumors
[158] and is approved for the treatment of hepatic encephalopathy
[160].
3.2.2. Inhibition of TLR2 and TLR4
Synthetic analogs derived from the lipid A portion of LPS (E5564 and CRX-526) inhibit TLR4 by preventing LPS binding to the TLR4–MD2 complex. The intracellular domain of TLR4 is targeted by another TLR4 inhibitor, TAK-242. Despite their inhibitory action on LPS-induced inflammation, they have not been tested for cancer prevention in either clinical trials or animal models
[161][162][163]. Similarly, OPN305, a humanized monoclonal antibody, reduces in vivo inflammation but has not been tested regarding cancer prevention
[164].
There is a need to develop appropriate TLR-targeting drugs for the prevention/elimination of cancer. Several types of drug molecules exist based on their biochemical nature such as protein, small molecules, and aptamers. Each of them has its own benefits and drawbacks which affect physicochemical and pharmacokinetic properties of the associated drug. Discovering an appropriate drug is quite a lengthy and complex process which involves target selection and its validation; compound screening and lead optimization; preclinical studies; and clinical trials. Target selection and library screening involve various computational approaches such as analysis of genome and proteome; high-throughput screening; virtual screening; and combinatorial chemistry. These traditional approaches are time-consuming and expensive. It would be a better choice to use state-of-the-art and most advanced approaches like artificial intelligence (AI) for the screening of TLR-targeting compounds. More efficient and specific drug will lead to better and safe prevention of TLR-associated cancers with fewer side-effects.