1000/1000
Hot
Most Recent

The mining of heavy metals from the environment leads to an increase in soil pollution, leading to the uptake of heavy metals into plant tissue. The build-up of toxic metals in plant cells often leads to cellular damage and senescence. Therefore, it is of utmost importance to produce plants with improved tolerance to heavy metals for food security, as well as to limit heavy metal uptake for improved food safety purposes.
“Heavy metals” as a term has been widely debated and criticized. However, Duffus[1] reviewed the concept and proposed that the scientific world come up with a standard definition. Heavy metals occur naturally in the Earth’s crust[2] and are extensively mined in various parts of the world. This practice has hugely contributed to the uncontrollable anthropic inputs into the environment. These heavy metals have a very long half-life, and therefore persist in the environment for extended periods because they cannot be degraded[3][4]. Therefore, they can accumulate and exert toxic effects on microorganisms, plants, animals, and humans. Heavy metals have become a major environmental issue, and if the current environmental contamination persists, then heavy metal pollution will also be a great concern for the future[5].
Heavy metals such as arsenic, cadmium, chromium, lead, and mercury rank among the most toxic metals, with high public health significance. Toxicity in humans and animals often depends on several factors, such as quantity, route of exposure, and chemical species. However, age, gender, genetics, and nutritional status also play key roles in physiological responses to heavy metals. Humans and animals can come into contact with heavy metals through rocks, soils, water, and the atmosphere[6]. Plants utilize all of the mentioned resources to survive, and due to a sessile nature cannot avoid contaminated environments. Therefore, these plants take up the heavy metals and sequester the metals in various tissues, maintaining heavy metal concentrations below toxicity levels[7]. Humans and animals which consume heavy metal contaminated plants can accumulate them to toxic levels in various organs[8]. Therefore, it is important to understand the pathways that control heavy metal uptake in plants. This will assist in regulating tolerance to, and monitoring toxicity of, heavy metals in order to limit the risk that results from the consumption of plants contaminated with heavy metals.
Plants have evolved complex signaling mechanisms that regulate their responses to heavy metal stress[9]. These mechanisms include the “universal” cascade, which consists of reception (stimuli perception), transduction (intra and extracellular signal amplification), and response (enzymatic or non-enzymatic) steps[10]. Therefore, in this review, we focus on some of the key events at each step of the plant signaling cascade under heavy metal stress. We discuss the importance of the apoplastic space in the reception of heavy metals, especially the roles of the cell wall and the plasma membrane (PM). We discuss the complexity of reactive oxygen species (ROS) signaling in plants under heavy metal stress. We review and discuss the key importance of glutathione and hydrogen sulfide, in heavy metal detoxification, as well as serving as possible heavy metal stress signal amplification molecules. In addition, we also examine the latest US Environmental Protection Agency[11] data about key heavy metals, in order to understand toxicity for food safety purposes by analyzing the oral reference doses (RfDo) for the heavy metals (Table 1).
Table 1. Elements classified as heavy metals according to a density at room temperature of more than 5 g/cm3. The values for the limit in residential soils and oral reference dose were obtained from the U.S. Environmental Protection Agency (USEPA) for June 2020[11]. The density at room temperature values was obtained from Shackelford et al. [12]. The abundance rank of the elements in the Earth’s crust was extrapolated from Anderson[13].
| Elements | Limit in Residential Soils (mg/kg) | Reference Dose (RfDo) (µg/kg/day) | Density at Room Temperature (g/cm3) | Abundance Rank |
|---|---|---|---|---|
| Antimony (metallic) | 31 | 0.40 | 6.70 | 62 |
| Arsenic | 0.77 | 0.30 | 5.73 | 55 |
| Cadmium (Diet) | 78 | 1 | 8.65 | 64 |
| Chromium (VI) | 0.31 | 3 | 7.19 | 21 |
| Cobalt | 23 | 0.30 | 8.90 | 31 |
| Copper | 3100 | 40 | 8.96 | 25 |
| Iron | 55,000 | 700 | 7.87 | 4 |
| Lead | 400 | N/A | 11.34 | 36 |
| Manganese (Non-diet) | 1900 | 24 | 7.47 | 12 |
| Mercury (elemental) | 11 | N/A | 13.53 | 66 |
| Molybdenum | 390 | 5 | 10.28 | 54 |
| Nickel (soluble salts) | 1600 | 20 | 8.91 | 23 |
| Silver | 390 | 5 | 10.49 | 65 |
| Thallium (soluble salts) | 0.78 | 0.01 | 11.85 | 60 |
| Tin | 47,000 | 600 | 7.31 | 48 |
| Tungsten | 63 | 0.80 | 19.25 | 53 |
| Uranium (soluble salts) | 16 | 0.20 | 19.05 | 50 |
| Vanadium | 390 | 5 | 6.11 | 19 |
| Zinc | 23,000 | 300 | 7.14 | 24 |
| Zirconium | 6.30 | 0.08 | 6.51 | 18 |
Roots serve as the main point of entry through which metal elements enter the plant system[14]. The heavy metals in the soil enter the plant root by freely diffusing through the cell wall in an unregulated manner[15]. Therefore, in the roots, this is the initial barrier for metal contact. Furthermore, the cell wall is mainly composed of cellulose, hemicellulose, and pectins[16]. The cell wall has the ability to bind heavy-metal ions in negatively charged sites (–COOH, –OH, and –SH), which results in the alteration of cell wall composition[17]. These alterations cause damage to the cell membrane, particularly to the PM. Consequently, the disruption of membrane integrity is believed to be a result of complex interactions involving functional groups of the membranes and heavy metals[18]. This is the primary site for signal perception, the downstream trigger of the defense response and the cell fate decision under heavy metal stress[19][20]. Liu et al.[21] conducted a proteomic study on Elsholtzia splendens cell walls under copper stress, and observed that ~40% of the differentially expressed cell wall proteins (CWPs) showed higher abundance in response to copper stress. These proteins are involved in antioxidant defense, cell wall polysaccharide remodeling, and other metabolic processes. Furthermore, the study showed that ~60% of the CWPs were in low abundance in response to copper stress, and that these proteins were involved in signaling, energy, and protein synthesis. The study identified Hsp70, small G-protein, and RAS-related GTP-binding proteins, which were all responsive to the copper stress, and which have essential roles in signal transduction. Even though the study by Lui et al.[21] clearly showed the potential role of the cell wall in downstream signaling responses under heavy metal stress, Parrotta et al. [22] stated that the intricate signaling mechanisms of the cell wall in heavy-metal responses are not well understood. Parrotta et al.[22] then highlighted the potential roles of aquaporins and wall associated kinases as two potential future targets for studying the cell wall signaling component under heavy metal stress. Indeed, Przedpelska et al. [23]observed gating of aquaporins within 10 min of heavy metal application to onion epidermal cells, irrespective of the metal applied (zinc, lead, cadmium, and mercury). The importance of aquaporins in the signaling response to heavy metals was also confirmed by Ariani et al. [24], who studied AQUA1 (a mercury-sensitive aquaporin). The results showed that a high concentration of zinc down-regulates aqua1. The zinc response caused the re-localization of aqua1 into newly formed pro-vacuoles through the regulation of intracellular trafficking and post-translational modifications.
Secretory vesicles are the components that transport constituents required for the construction of the cell wall. These vesicles congregate beneath the PM, and are subjected to fusion with the PM[17]. In all plant cells, intracellular transport is vital for the distribution of membrane-bound vesicles and organelles to their respective cellular destinations. Furthermore, vesicle trafficking is important for the organization of endomembranes. A disturbance in the vesicular trafficking system under heavy metal stress can lead to the disruption of cell wall construction, ultimately resulting in inhibition of cell growth[14]. Hence, it is important to understand how plant vesicular trafficking systems are influenced by heavy metal stress and how this regulation alters the cell wall signaling component. Nonetheless, vesicle-bound molecular motors are the driving force of vesicle trafficking within plant cells. These molecular motors interact with the cytoskeletal elements, microtubules, and specifically, actin filaments (localized movement of vesicles). These cytoskeletal elements are recognized as the tracks for vesicle transport. Fan et al.[17]used Arabidopsis thaliana to understand the significance of the cytoplasmic calcium gradient alongside actin filaments (AFs) in the vesicular trafficking system within root hairs. A. thaliana root hairs were subjected to cadmium treatment and fluorescence labelling with FM4-64 dye to track the impact of cadmium on endocytosis potential and membrane recycling (Figure 1). Fan et al. [17] showed that both endocytosis and vesicular trafficking were disrupted. To understand what influenced this disruption, the authors proceeded with in vivo labelling, along with laser scanning confocal microscopy, to examine the effect of cadmium on actin organization. The authors observed that the usual longitudinal arrangement of the actin filaments was altered towards a transverse arrangement, and that this alteration interrupted the vesicular trafficking system. Perfus-Barbeoch et al. [25] observed that cadmium enters the root hairs via calcium-selective channels, because cadmium has an identical charge and ionic radii to calcium. Zhang et al.[26]stated that calcium concentrations are crucial in actin filament organization, as calcium binds to gelsolin-like proteins (a calcium-dependent actin severing protein), thus activating the gelsolin, and promoting the disruption of actin filaments. Consequently, as cadmium mimics calcium, the cadmium within the cells binds to gelsolin to promote actin filament destabilization and depolymerization. Since the study revealed that the introduction of cadmium (1) disrupted the calcium gradient within the cells, (2) induced actin filament depolymerization, and (3) demonstrated a decline in vesicular trafficking, Fan et al.[17] noted that vesicle trafficking in the A. thaliana root hairs, were to some extent dependent on the calcium gradient and actin filament arrangement. Callose deposition at the cell wall is regarded as a defense mechanism after plants sense cadmium stress[27], and the first deposits of callose in plants were observed 5 min to several hours after contact with an inductor [28]. Therefore, because the callose synthase (CESA) is assembled primarily at the Golgi apparatus[17], the depolymerization of the AF changes the conformation of CESA and ultimately inhibits the deposition of callose in the cell wall, rendering this defense mechanism inadequate. Thus, plants require a mechanism to limit this effect of cadmium or other heavy metals on actin filaments to ensure proper callose deposition at the cell wall.
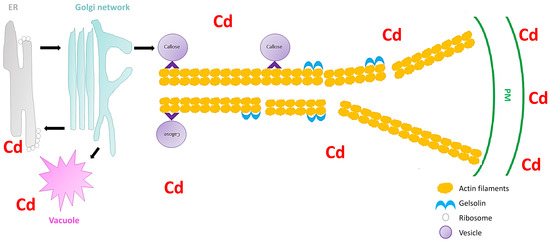
Figure 1. Schematic image showing the severing of the actin filaments by gelsolin under cadmium stress in Arabidopsis thaliana (extrapolated from Fan et al.[17]). The vesicles move (black arrow) between the endoplasmic reticulum (ER), Golgi network, vacuole, and the plasma membrane (PM). Cadmium stress triggers a callose response which are sorted and packaged in vesicles for unloading at the PM. The vesicles attaches to the molecular motors (^) which facilitate the movement on the actin filaments. However, cadmium also activates gelsolin, which cleaves the actin filaments, and therefore the vesicles cannot reach the PM and unload the callose.
A role for pectin in the cell wall signaling cascade via the receptor-like kinase, FERONIA, was proposed by Yang et al.[29]. However, the mechanisms for pectin sensing and transducing of wall signals under heavy metals remain undiscovered. Therefore, understanding the plant vesicular trafficking events that modulate pectin at the cell wall could be a starting point to understand the involvement of pectin in cell wall signaling under heavy metal stress. Krzeslowska et al. [30] studied the mechanism of internalization of pectins in the tip of a growing apical cell protonemata of Funaria hygrometrica under lead stress. The authors studied the pectin epitope JIM5 (JIM5-P) because lead has a high affinity for this pectin epitope[31]. To study the internalization and vesicular trafficking of lead, Krzeslowska et al.[30] employed FM4-64 dye, and observed that vesicular trafficking intensification and common internalization of JIM5-P from the cell wall was as a consequence of lead accumulation. Furthermore, by employing the immunogold labelling method for JIM5-P identification in transmission electron microscopy, the authors observed that in the control plants JIM5-P occurred mainly within the cell wall. However, under lead stress the JIM5-P was internalized and mainly detected within PM invaginations and in different sizes of vesicles [29]. This pectin–vesicle trafficking signaling response was also observed in other plants, which could suggest that this response is a universal lead stress response[32]. The immobilization of heavy metals to cell walls via vesicle trafficking could be an important downstream plant cell tolerance reaction to stress responses. Furthermore, the internalization of these vesicles can also be internalized into endocytic invaginations of the PM, and could be transported via the endocytic or secretion pathway within plant protoplasts.