1000/1000
Hot
Most Recent

The pH sensors are gaining widespread attention as non-destructive tools, visible to the human eye, and are capable of real-time and in-situ response.
Optical chemical sensors are considered “eyes capable of seeing beyond the human perception” in that offer advantages to human sight and can be regarded as miniaturized real-time analytical devices [1]. Optical sensors represent great analytical tools for technologies requiring real-time continuous monitoring and gained researchers’ interest in many chemical contexts. They are routinely used for biological, environmental, and medicinal applications [1][2].
Optical transduction techniques are a consequence of the selective change of their optical properties on interaction with the analyte. Absorption and fluorescence emission spectral patterns are among the most explored optical properties. Spectral absorbance is defined as the logarithm of the ratio between transmitted spectral radiant power through a material and the incident radiation. Absorbance spectroscopy is an analytical technique based on the measurement of the absorption of radiation across the electromagnetic spectrum as a function of the wavelength of radiating light. The result is called the absorption spectrum of the sample. On the other hand, when irradiated with exciting radiation, a chemical compound can produce its own emission spectrum. Emission is the electromagnetic radiation produced by the electrons of the molecule when transitioning from a higher energy state to a lower one. There are several possible electron transitions for each atom/molecule, with specific energy difference that represent the emission spectrum. Fluorescence emission occurs to relax an excited singlet state of an atom/molecule, upon absorption of a radiation, to a ground singlet state and the overall spin is conserved. Upon returning to the ground state, the excited atom/molecule emits a photon of lower energy and longer wavelength than the absorbed photon. The radiation used to promote the emission is called “excitation” wavelength, while the resulting radiation is known as “fluorescence”. Typically, the excitation wavelength is represented by the absorbance peak wavelength. The difference between the “excitation” and the “fluorescence” peak is known as “Stokes-shift”. Large Stokes-shifts improve fluorescence signal due to a decreased overlap between the absorbance and the emission bands [3]. Fluorescence and absorption spectroscopy can be considered complementary techniques and provide a response in the visible spectral region and are employed as analytical chemistry tools to quantify the amount of a particular analyte in the sample [4]. The first stage of optical sensor transduction involves a chemical interaction between the analyte and a transductor to produce an optically detectable signal. Substances able to produce absorbance/fluorescence spectra in the visible region up to near-infrared (NIR) region are typically named chromophores/fluorophores [5][6]. These substances are organic or hybrid molecules with an electronic configuration capable of transitions in the visible spectrum. For our aims, we focus on sensors producing optical signals within the visible spectrum and detectable in absorbance and/or in fluorescence. Specifically, we refer to colorimetric sensors as a class of optical sensors able to change their color when stimulated with natural light, and fluorescence sensors able to change intensity and/or emission color when stimulated with UV-light [7]. Among colorimetric sensors, we refer to “visual sensors” (abbreviated as VSs) since, similar to an “optical instrument”, the eyes are able to detect the signal produced by these molecules [8][9][10][11]. These sensors offer an advantage over spectrophotometric and electroanalytical analysis, more expensive and time-consuming [12][13]. VSs can offer real-time and in-situ sensing, even in remote sensing mode, and non-destructive analysis. Typically, VSs are classified into on-off and gradual sensors, respectively [14][15][16][17]. For on-off VSs, two different colors or different emissions are detected between low and high pH values [17]. For gradual VSs a progressive colorimetric/fluorimetric response is obtained corresponding to an increase of the analyte concertation (Scheme 1) [18][19]. If the sensor emission can be stimulated by two excitation wavelengths, it will be referred as “ratiometric” sensor [20].
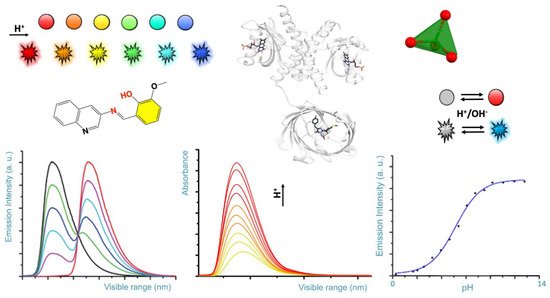
Scheme 1. Rendition of typical colorimetric variation of pH sensors (colored spheres), and fluorescence emission as a function of the pH (colored flashing shapes). Typical, spectral diagrams observed for the emission and absorbance as a function of the pH and for a given wavelength are represented on the bottom.
Hints from history help us to understand how visual pH sensors research is evolving. In 1907 Henderson published a paper describing the relation between the concentration of hydrogen ion [H+] and buffer composition. In 1909, Soren Peder Lauritz Sørenson proposed the more convenient pH and pKa definitions as the negative logarithm of [H+] and of the equilibrium constant, respectively. Although Henderson defined the equilibrium constant, Ka, in terms of a concentration ratio in 1908, it was in 1916 when Hasselbalch proposed a recognized relationship between pH and pKa known as the Henderson-Hasselbalch equation. This equation relates the two parameters to the equilibrium concentrations of a dissociated acid [A−] and its non-dissociated acid form [HA], respectively:
pH = pKa + log ([A−]/[HA])
This equation allows to deduce pH sensor ability of a molecule by knowing its Ka value. Sørenson pairing of a hydrogen electrode with a calomel reference electrode was followed by the 1920 Duncan McInnes and Malcolm Dole first glass electrode, equipped with a glass semipermeable membrane. The potential of a hydrogen electrode (proportional to “H+ activity”) dipped into the analyte solution represents the signal that is correlated to the measured pH value.
Since the first pH measurement in 1909 by Sørenson, the development of more selective sensors of proton H3O+ (simply H+) concentration in solution still fascinates chemists [21]. From the earliest pH sensors to modern pH chemosensors over a century has passed. Today, we recognise pH as a fundamental parameter of both chemical and biological interest. Enzymatic reactions, as well as overall cellular buffer systems, function within a narrow and specific pH range. Deviation from this value can help to diagnosis the acidic environment of cancer cells [22]. Therefore, monitoring of pH within biological systems represents a great goal for the ongoing “pH sensors” research and it is not surprising the growing researchers’ interests in design, synthesis, chemical characterization, and sensing mode. A preliminary stage for sensor development involves custom-made sensors, not necessarily targeted for biological purposes. These sensors can be used as starting point for further modification of functionally sensing needed for biological and biomedical use. Today, the scientific frontier is represented by bio-engineered, nanosized, and multi-channel systems capable of non-invasive on-site analysis.
Despite several studies encompassing the topic of visual pH sensors, we report an overview of the most recent types of developed visual pH sensors considering different typological and chemical entities and three-dimensional structures. In this review, we focus on the state-of-the-art of visual pH sensors (abbreviated as pH VSs) developed over the past five years (years 2016–2021) based on synthetic novelty, study completeness, optical selective response, theoretical deepening, and biochemical applications. Specifically, we review the literature and organize the discussion around pH VSs in the following sections (also represented in Scheme 1):
(1) Molecular synthetic organic sensors;
(2) Metal organic framework (MOF)-based sensors;
(3) Sensors from engineered nanomaterials;
(4) Bioengineered sensors.
Several mechanisms explain the fluorescence, including photoinduced electron transfer (PET), internal charge transfer (ICT), Förster (or fluorescence) resonance energy transfer (FRET), excited-state intramolecular proton transfer (ESIPT) or other formation of excited states able to produce photon emission [21][23][24][25].
Photoinduced electron transfer (PET) is an excited state electron transfer process occurring in the same molecular system and as a consequence of an electron transfer from electron-donor to an acceptor-donor atom via a non-radiative pathway. PET process can quench fluorescence signal of an organic molecule. In many sensors, PET process channels signal to the analyte that in turn results in an emission of fluorescence [26].
Intramolecular charge transfer (ICT) is a mechanism occurring for fluorescent sensors when an electron-donating group of the fluorophore sensor is linked to an electron-acceptor group. During sensing processes, the sensor electron density is greatly affected by the analyte resulting in significant shifts in the absorption and fluorescence emission bands. Molecules with enhanced acidity or basicity in the excited state undergoing an intramolecular excited-state proton transfer (ESIPT) process in presence of the analyte are ideal candidates as pH sensors.
Energy transfer by resonance (FRET, Fluorescence or Förster Resonance Energy Transfer) represents an energy transfer between fluorophores. FRET is useful to obtain structural information of biological molecules and this spectroscopic technique allows to identify the distance between two molecules with extreme precision. FRET mechanism explores the mutual interference of fluorescent molecules, called donor and acceptor, bound to the biological molecule of interest [27]. The donor molecule such as a pH sensor can be excited with a specific wavelength and its emission energy in turn can be transmitted to the acceptor molecule. FRET energy transfer can occur if the molecules are in close proximity. Finally, if the acceptor molecule gives an emission, it can be measured by the operator.
Early traditional pH sensor molecules were extracted from flowers, fruits, and vegetables as sustainable and non-toxic natural dyes (colored molecules). For example, an efficient colorimetric pH sensor based on anthocyanin was recently extracted from purple sweet potato [28][29]. These established sensors were followed by novel synthetic chromophores/fluorophores functioning as pH sensitive compounds. These molecules meet specific analytical requirements such as photostability, water-solubility, large lifetime, Stokes shifts, and fluorescence quantum yields. The newest synthetic sensors are often guided by logic of low-cost process, environmental sustainability, and biocompatibility.
The first step for the development of novel pH VSs, is represented by the synthesis of simple small organic molecules or organic polymeric systems. These pH VSs can function in an on-off, or in gradual and/or ratiometric mode. For analytical purposes, especially in aqueous solution, on-off pH sensors provide a rapid and sharp change of color around a precise pH value that allows for pH monitoring. On the opposite, for many biological samples, where a gradual and small change around physiological pH (or a different value) is experienced, gradual and/or ratiometric pH VSs are more suitable [30][31][32]. Ratiometric sensors allow for measurements of two emission wavelengths in order to calculate the ratio of fluorescent intensities for a high measurement accuracy [33]. This ratio has the advantage of being independent by the total fluorescence intensity, making the signal correction for optical interference (readout system, photobleaching, inhomogeneity) no longer necessary and therefore, of easier and immediate application. A discussion of molecular organic pH VSs will follow in Section 3.1.
Electrochemical pH sensors development was achieved with significant results in last few decades for the measurement of potential, current, or impedance of a selective electrode. These are primarily made of inorganic materials (e.g., glasses), or in combination with nanomaterials (e.g., doped membranes) [34][35]. On the opposite, low and high molecular weight organic chemosensors are designed for measurements of pH value and utilize easily exchangeable protonation/deprotonation equilibria (or metal ions) of nitrogen and oxygen atom groups [36]. These sensors can also measure the pH of biological samples including the pH value of subcellular locations on different scale [37]. Tunable chemical fingerprint and sensing mechanisms can be achieved by chemical branching modification of a pH sensitive dye core’s molecule fragment. Polymeric pH VSs, obtained by linking of several sensing organic molecules, used as monomers, can be fixed to a material of support and are ready for potential functional applications. In this section, we discuss the recent well characterised molecular organic VSs functioning in colorimetric and/or fluorescence mode, by on-off or gradual and/or ratiometric response [38][39]. We examine their chemical mechanisms and provide considerations for their potential use. A selection of synthetic pH VSs is summarised in Figure 1 (boxes 1–5) and is grouped based on common visual response and/or chemical features. For a quick glance, nitrogen and oxygen atom groups responsible for accepting or donating hydrogen atom for protonation/deprotonation are marked in red, and their linked chemical ring(s) marked in yellow. Sensor colorimetric response is represented by colored spheres (see Figure 1) and corresponding fluorescence emission colors (as visually experienced) is represented by flashing shapes.
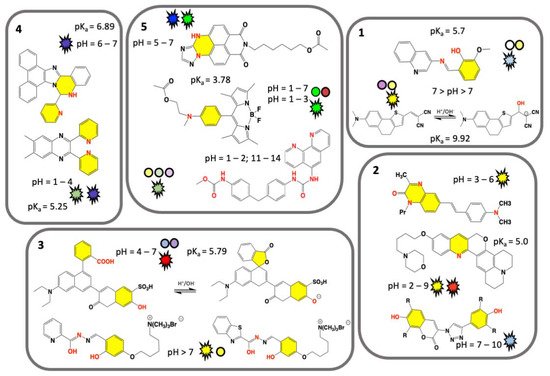
Figure 1. Chemical structures of recent synthetic molecular organic visual pH sensors grouped on the base of a common visual response or chemical features. Nitrogen and oxygen atom groups responsible for protonation/deprotonation are marked in red, and their chemical ring(s) marked in yellow. Sensor colorimetric response and pH value are represented by a colored sphere, while fluorescence emission by a flashing shape. Color matches to the sensor real color within the displayed pH range. Unique value of pKa is displayed. Box 1. Classical colorimetric and fluorescent pH VSs [22][40]. Box 2. Predominant fluorescent pH VSs [41][42][43]. Box 3. Designed water-soluble pH VSs [44][45][46]. Box 4. AIEgens pH VSs [47][48]. Box 5. Polymeric pH VSs [49][50][51][52][53]. Adapted from references indicated in square brackets.
Two classical pH VSs are illustrated in Figure 1 (box 1) producing absorbance and fluorescence colorimetric response from acidic to basic medium. A pH VS was chemically synthesised by condensation of 3-aminoquinoline Schiff base with o-vanillin and fully characterized by A. Saha and coworkers [22]. Obtained as an “easy-to-make” organic sensor, it functions as an acidic pH fluorescence and colorimetric sensor. Colorimetrically the sensor exhibits two bands at 330 nm and 400 nm at pH 7.0, associated to n-p* and p-p* transitions, respectively. The intensity of the absorption band at 330 nm gradually increases with decreasing of pH from 7.0 to 2.0, while a concomitant decrease of the absorption band at 400 nm is measured. As for the fluorescence response, the sensor exhibits naked-eye perceivable color change from acidic to basic pH under a UV-lamp (Figure 1, box 1). The fluorescence is quenched by PET mechanism resulting from protonation of the imine nitrogen group. Deprotonation of the same nitrogen cause locking of PET mechanism which in turn results in restoration of fluorescence with a neat on-off performance [22]. This sensor gives-off a fluorescence signal change between normal and cancer cells, as function of pH in vitro cytotoxicity [22]. A similar colorimetric and fluorescence turn-on pH sensor is comprised of a highly colorimetric naphthalenone scaffold (Figure 1, box 1) and studied by S. Wu and coworkers [40]. This sensor shows a color change from light purple to yellow (acid to basic), and a fluorescence signal in the pH range of 9.0–14.0, with an emission from colorless to orange, and finally to yellow (box 1) [40]. This response is associated to an ICT mechanism induced by structural changes caused by -OH group binding, which translate a non-fluorescent molecule into a highly emissive form. Fluorescence imaging of HeLa cell line reveals a good cell membrane permeability, and its ability for selective monitoring even of extreme basic pH variation [40].
Predominant fluorescent pH VSs are illustrated in Figure 1 (box 2). A series of six quinoxalin-2-ones dyes with N,N-dialkylaminostyryl substituents in different combinations were studied by T.P. Gerasimova and coworkers [41]. For pH-sensing in aqueous solution the dyes were encapsulated into L-α-phosphatidylcholine based bilayers. pH sensitive emission reveals a significant effect of the dyes structure/interaction in the bilayer and produces a maximum sensing response within pH range of 3.0–6.0. Quantum chemical calculation revealed that the protonation mechanism undergoes different paths depending on the alkyl group of the N,N-dialkylaminostyryl substituent and/or the position of a donor substituent relative to the quinoxalin-2-one [41]. A lysosome-targeting sensor (named CQ-Lyso) based on the chromenoquinoline chromorphore gave ratiometric fluorescence response to intracellular acidic pH in living cells (box 2) [42]. This sensor was developed as a pH VS: in acidic media, the protonation of the quinoline ring induces an enhanced intramolecular charge transfer (ICT). This process results in a yellow fluorescence in neutral/basic media (pH 7.4), and a change from yellow to red/brown color using the same excitation wavelength at pH 4.0 [42].
A limited number of pH sensors with an off-on-off fluorescence signal variation have been reported to date, potentially more useful than conventional on-off sensors. Sensors with off-on-off behavior can detect a selected pH range within the whole pH range in which their signals are visible (-on state). Among off-on-off fluorescence sensors, the one consisting of a coumarin group fused to a triazole ring was developed (box 2) by T. Hirano and coworkers [43]. This sensor can be used for fluorescence imaging of pH within intracellular human mcf-7 cell line (cancer cell) and it turns fluorescent at pH 6.0, but not at pH 8.0; suggesting a use in microenvironment living cells [43].
Specifically designed water-soluble pH VSs are illustrated in Figure 1 (box 3). Water solubility is a key requirement for biological applications and water-soluble visual pH sensors are uncommon and highly required. To obtain a water-soluble sensor specific ionic groups such as sulphonic or quaternary ammonium groups are attached to the sensing molecule. A water-soluble pH sensor for sensing and imaging was obtained by introducing a sulfonic group to the chemical structure of condensate rings by A. Zheng and coworkers [44]. The synthesized sensor contains a pH-responsive phenol group undergoing deprotonation (box 3), that in turn improves the ICT mechanism [44]. In basic condition an intramolecular spiro-cyclization and decreased of π-conjugation of the sensor occur. As a result, the maximum absorption of the system is red-shifted, and the fluorescence is enhanced. The sensor can be used for pH sensing through its absorption or fluorescent signals, and a linear relationship was detected. Fluorescence imaging and cell viability of human cancer HepG2 cell line in buffer with pH between 4.5 and 7.4 show a clear fluorescence enhancement [44].
Sensing ability based on small water-soluble, simple, and organic molecules attracts researchers’ interest [45][46]. By adding on the sensor’s core a flexible five methylene groups branch bearing a charged trimethylammonium bromide group (box 3) B. Panunzi and coworkers developed two water soluble sensors, while preserving their organic phase solubility. These sensors are based on a substituted aroyl-hydrazide skeleton undergoing to tautomeric equilibria depending on pH value. In these molecules colorimetric effect is the result of ESIPT mechanism. Owing their amphiphilic nature, the charged sensors could potentially interact with cell membrane without altering the bilayer organization [40]. These sensors show naked-eye on-off switch at neutral pH, absorption and fluorescence response, high sensitivity.
Beginning with the pioneering studies of Tang and Park in 2001 a unique class of molecules called AIEgens (aggregation-induced emission) was developed [54][55]. Typically, AIEgens molecules contain multi-aromatic conjugated rings with potentially free rotating chemical groups. Although weakly or not emissive as isolated molecules, they become emissive in the solid state, or in concentrated solution, due to intermolecular processes and as consequence of their restriction of intramolecular rotation (RIR) [55]. AIEgens molecules have shown properties as fluorophores and exhibit aggregation-induced emission, or emission enhancement. The aggregate state of the AIE molecules, often characterized by X-ray structural studies, is the result of hydrogen-bonding, p-stacking, or other Van der Waals interactions [55]. These unique characteristics differentiate AIEgens from conventional luminophores and are currently explored as optical sensors including specific interactions derived by samples pH changes. Recent examples of AIEgens pH VSs are illustrated in Figure 1 (box 4). A visual AIEgen pH comprised of a (2-pyridyl)-quinoxaline dye with two free twisting pyridyl rings in 2 and 3 positions of the quinoxaline moiety sensor was developed by A. Misra and coworkers (Figure 1, box 4) [47]. The molecule exhibits an AIE enhancement triggered by proton addition. As a result, the sensor solution change from colorless to blue color in the aggregate state and under UV lamp. Furthermore, protonation causes a reversible fluorescence switch between basic and acidic conditions and the emission color changes from blue to green in the aggregate hydrosol state (aka a colloidal mixture having water as the dispersion medium). The blue shift is observed in both absorption and emission spectra and theoretical calculations served to deduce the mechanism. A more recent AIEgen visual pH sensor is represented by the phenanthro-imidazole-based sensor for dual-responsive turn-on detection of acidic pH by Q. Deng and coworkers (box 4) [48]. This sensor is also able to recognize copper ions. The weakly fluorescence sensor exhibits typical AIE turn-on phenomenon in water/ethanol mixture. The sensor was successfully employed in an aggregate form for acidic pH turn-on in HeLa cells, proving to be useful for monitoring cellular pH.
Trending methods of pH detection involve polymeric materials, widely explored for biomedical and environmental applications [56]. Comprehensive review papers are available and discuss organic micro- and macro-scaled organic pH sensors including healthcare applications [57]. In most cases pH sensing involves electric and electrochemical methods [57][58]. However, there are only few polymeric pH sensors exploring absorption and fluorescence response. Many polymeric pH VSs are obtained by immobilization/entrapping of dyes on materials including silicate, polyvinylidene fluoride (PVDF), cellulose acetate [59]. One approach is represented by the use of a fluorescent polymeric micelle, consisting of a pH sensitive fluorophore linked to a fatty acid molecule, and able to self-assemble in a vesicle-like envelope inside the cell, able to sense a pH variation [60]. On the other hand, polymeric pH VSs can be obtained by covalent linkage of fluorophores/chromophores through several strategies of polymerization or copolymerization [61][62][63]. A representative polymeric pH VS was developed by Y. Tian and coworkers in 2013 by copolymerization of monomeric units of fluorescein (green color) and/or dihydrofuran (red color) in methacrylate and acrylamide copolymers and used as a biocompatible material for drug delivery, bioimaging, and biosensing [64]. Recently developed polymeric pH VSs are illustrated in Figure 1 (box 5).
A classic pH sensor containing five monomeric units of triazole heterocycle fused in a five-condensed rings unit, and conjugated to a methacrylate and acrylamide copolymer, was developed by Y. Tian and coworkers [49]. This mixed sensor/copolymer functions as a weak electron-donor group in acidic conditions, while in basic conditions, the -NH group is deprotonated resulting in a stronger electron-donating ability of the triazole unit. The polymer optical property is the result of an ICT mechanism triggered by protonation [49]. The pH sensor copolymer shows a strong fluorescence-based ratiometric pH response, exhibiting blue emission around 477 nm in acidic conditions, and green emission around 507 nm in basic conditions. Fluorescence responsive fragments were prepared as monomers which were polymerized to form a fluorescence responsive polymer. The polymer has the potential for monitoring cellular pH value as well as pH distribution in imaging techniques.
A different approach was used by H. Lee and coworkers to develop a polymeric pH fluorometric chemosensor containing a monomeric unit of difluoroboron dipyrromethene (Figure 1, box 5) [50][51]. This sensor exhibits excellent solubility and stability in water, and in acidic conditions shows an intensity enhancement of 4.6 times with respect to the unprotonated sensor. This characteristic is a consequence of PET suppression mechanism due to electron-donating of the sensor’s tertiary amine to its difluoroboron dipyrromethene skeleton group [51]. The response in acidic pH, was measured by fluorescence imaging for in vitro cells and E. coli bacteria (surviving in acidic pH) and paper strip embedded with the polymer was used to test practical in-situ applications.
A highly sensitive and pH-responsive polymeric system (named Phen-MDI-CA) represented by a chemical engineered sensor on a natural substrate was developed by J. Zhang and coworkers [52][53]. Specifically, cellulose was used as a skeleton, urea group as an anchoring bridge, and phenanthroline group either as a chromophore or a metal ion coordinating group. The Fe(II) cations can act as cross-linking agents among cellulose polymeric chains. Therefore, Phen-MDI-CA functions as sensor for iron cations while being responsive in a wide pH range [52]. Colorimetrically, it discriminates within pH range 11.0–14.0 on paper stripes test, and pH 1.0–2.0 by fluorescence [53]. Three ratiometric systems were obtained by mixing Phen-MDI-CA with pH-responsive dyes used as a reference, consisting of meso-tetraphenylporphyrin, a protoporphyrin derivative, and malachite green in DMSO. Specifically, these ratiometric mixtures display different emission colors within a small range of 0.2–0.4 pH units within the overall pH ranges of 11.0–14.0 and 1.0–2.0. Therefore, these ratiometric systems amplify color difference allowing for a good measurement accuracy.
An interesting class of polymeric pH VSs is represented by nucleic acids and can be targeted for pH sensing and combined to dye molecules to form pH sensors. One approach is based on the use of functionalized short single-stranded nucleic acid sequences (30–50 bases) of RNA or DNA able to form hairpin structures upon annealing with a complementary oligonucleotide chain. By covalently linking a pyrene group to both 5′ and 3′ ends of an oligonucleotide B. Juskowiak and coworkers were able to form a so-called molecular beacon (MB). This dual-labelled oligonucleotide was used as a pH fluorescent sensor because emitting in acidic pH (emission ~ 480 nm) [65]. When pH decreases, protonation of cytosine nucleotide causes a switch to a folded tetraplex structure. As consequence, MB sensor resulted in an efficient reversible DNA-based switch for real-time intracellular pH monitoring of live HeLa cells.