1000/1000
Hot
Most Recent

Lung transplantation is the gold standard treatment for end-stage lung disease. Lung transplantation helps patients with end-stage lung disease due to a number of causes such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, sarcoidosis, interstitial lung disease (ILD), pulmonary fibrosis, and pulmonary hypertension. Primary graft dysfunction (PGD) is a form of acute lung injury that can occur after transplantation within the first 72-h. At a tissue level, PGD is the result of diffuse alveolar damage. This damage manifests clinically as severe hypoxemia and lung edema with diffuse pulmonary infiltrates as seen on CXR. PGD is the leading cause of 30-day mortality post-LTx, affecting 11–25% of patients. The clinical gold standard for organ preservation is cold static preservation (CSP); however, ex-situ lung perfusion (ESLP) is a relatively novel donor lung preservation and reconditioning technology that has been shown to improve lung function and increase organ utilization.
The events preceding lung retrieval as well as those that occur during transportation and after lung transplantation, all contribute to the development of LIRI and PGD. In this sense, the logistics of lung transplantation are made up of a series of hostile environments. To better appreciate the risks to graft function during lung transplantation, it is helpful to examine the events in terms of donor-related/retrieval risk factors, transportation/CSP risk factors, and recipient/transplantation risk factors.
A number of pre-transplant and donor-related risk factors are associated with LIRI and the development of PGD [1]. Studies of transplant outcomes have shown that during the first 24 h post-transplantation, donor factors predominate [2] whereas recipient risk factors are more influential beyond 24 h [3]. Donor-related risk factors for PGD include those that are pre-existing/hereditary and acquired.
Pre-existing/hereditary factors include race, sex, advanced age, and smoking [2]. Advanced age is a risk factor in other solid organ transplants, and PGD significantly increases when donors are older than 32–45 years [4]. The most significant pre-existing donor risk factor for PGD is a history of smoking [5].
Acquired donor risk factors are numerous and include excessive blood transfusions, hemodynamic instability resulting from brain death, aspiration, pneumonia, trauma, and prolonged mechanical ventilation [2]. Thromboembolism and fat embolism increase the risk of PGD by 5-fold and 25-fold, respectively [6]. Clinical factors that often precede brain death such as loss of surfactant, platelet occlusion of the microvasculature during hypovolemic states, pro-inflammatory cascades, and upregulation of adhesion molecules all negatively affect pulmonary endothelial function, which is the primary target of LIRI [7].
A deleterious cascade of events surrounding brain death contributes to negative changes in the pulmonary vasculature. Braindead donors, or neurologic determination of death (NDD), donors are the most common donor for LTx. Brain death results in parasympathetic and sympathetic imbalance. This causes the release of inflammatory cytokines, hemodynamic instability with tachycardia, hypothermia, electrolyte derangements, and endocrine perturbations [2]. Donor hypotension and microvascular occlusion impair organ perfusion causing ischemia and compromising graft quality [8]. These effects are compounded by poor tissue oxygenation during warm and cold ischemia at the time of retrieval, triggering cell dysfunction, necrosis, and apoptosis [9]. Braindead donors are also prone to pulmonary injury from mechanical ventilation-induced barotrauma and alveolar recruitment maneuvers, despite the use of protective ventilation strategies. In sum, there are a number of consequences to graft quality as a result of brain death and the events preceding organ retrieval.
Explantation of the donor lung necessarily involves the complete cessation of blood supply (ischemia) and ventilation (hypoxia), and clinicians employ the use of cold temperatures and preservations solutions to mitigate the resulting injury. The complete cessation of oxygenation and blood supply is known as anoxic ischemia. This is characteristically different from ventilated ischemia, such as following a pulmonary embolism, where blood flow is interrupted but ventilation continues [10][11][12]. Therefore, lung retrieval and the associated LIRI are due to anoxic ischemia [13].
To limit the extent of LIRI during retrieval, surgeons employ several protective surgical and storage strategies. The most rudimentary strategy is an efficient operation that limits the duration of warm ischemia when the lungs are being surgically divided from their associated structures, including the heart and trachea. Prior to this step, most institutions flush the lungs antegrade through the pulmonary artery with a hypothermic, low-potassium, and dextran-containing solution. This solution composition has been established to produce superior graft function in animal transplantation models [14]. The total volume of the flush is around 60 mL/kg with PA pressures of 10–15 mmHg. Heparin and prostaglandin are administered ahead of the flush to prevent clot formation, decrease inflammation, and limit vasoconstriction and are associated with hypothermia and hypoxia [15][16]. Depending on the center an additional retrograde flush is often performed to remove any residual blood and clots. The lungs are clamped at moderate expansion (approximately 50% of total lung capacity) to maintain alveolar recruitment, optimize surfactant production, and provide a source of oxygen (albeit limited) during transportation [15][2][16]. Lungs are stored in a plastic bag containing additional preservation solution, then placed in a cooler full of ice. This strategy preserves the lungs at a temperature between 4 °C and 8 °C, which is considered protective for up to eight h. Preservation beyond eight h increases the risk of PGD significantly [15][2][16]. For this reason, ischemic durations beyond 8 h are a limitation to successful transplantation and must be factored into consideration prior to a transplant decision [15]. The aforementioned efforts to protect the lungs are helpful, but they do not protect the lungs from injury during the reperfusion phase at the time of transplantation [1].
There are a number of post-transplant and recipient-related risk factors associated with LIRI and PGD. Again, risk factors can be categorized as pre-existing/inherited and acquired. Pre-existing risk factors are similar between donors and recipients and include age, gender, and race [17][18]. Acquired recipient risk factors include elevated body mass index, pulmonary hypertension, idiopathic pulmonary fibrosis, sarcoidosis, underlying liver, and kidney disease, and heart failure, which are all associated with increased risk of PGD postoperatively. Elevated BMI portends the greatest increased risk among acquired risk factors, with BMI > 30 associated with an absolute risk increase of 11% for PGD compared to normal BMI controls [3][4][17].
Certain risk factors are associated with the transplantation surgery itself including the use of transfusions, elevated pulmonary artery pressures, and excessive mechanical ventilation. Transfusion-related lung injury (TRALI) is a known risk factor for PGD postoperatively resulting from intraoperative blood transfusions [18]. The manner in which reperfusion is carried out also influences PGD development. Research has demonstrated that pulmonary artery pressures should be increased gradually during reperfusion, particularly during the first ten minutes; hence, after completion of the vascular anastomosis, the PA clamps should be opened in phases over a ten-minute period or longer [19][20][21]. When cardiopulmonary bypass (CPB) is used for transplantation, the flow can be gradually increased in a similar fashion from the pump. As in the donor operation, mechanical ventilation can injury the lungs or exacerbate underlying injury leading to ventilator-associated lung injury and PGD [22]. For example, high tidal volumes and low positive end-expiratory pressure (PEEP) actually worsens lung function three h after reperfusion [23]. A common protective ventilation strategy is to gradually ventilate the lungs prior to unclamping the PA by targeting an FiO2 of 50%, PEEP of 5 cm H2O, and peak pressure of 20–25 cm H2O [24]. Recipient fluid overload, infection, and post-operative hemodynamic deterioration are also associated with PGD. These strategies are beneficial but do not eliminate the risk of PGD postoperatively.
The hallmark clinical features of PGD are poor oxygenation and diffuse pulmonary infiltrates on chest X-ray within 72 h post-transplantation [15]. Related features of pulmonary function are likewise worsened such as decreased pulmonary compliance, increased pulmonary vascular resistance, and the development of intrapulmonary shunts [25]. A rise in peak airway pressures makes adequate ventilation challenging. The combination of increased PVR and increased vascular permeability results in variable degrees of noncardiogenic pulmonary edema. In turn, there is progressive V/Q mismatch and poor oxygenation. In this way, PGD is similar to Acute Respiratory Distress Syndrome (ARDS) with regards to histology and clinical features [26][27]. PGD manifestations exist on a continuum of severity, which has been established by the ISHLT. Still, the diagnosis remains one of exclusion without specific diagnostic criteria [28].
PGD severity is qualified by the extent of hypoxemia with reference to the administered FiO2 (PaO2/FiO2 ratio) (Table 1). LIRI and PGD are associated with adverse outcomes including prolonged mechanical ventilation, increased post-operative length of stay, and increased mortality [7][29][30]. The presence of stage 3 PGD within 72 h is strongly associated with poor outcome [31]. This is in part related to the systemic consequences of LIRI that reach beyond the lungs and result in multisystem organ dysfunction.
Table 1. ISHLT PGD grading [32].
| Grade | P/F Ratio | Chest X-ray |
|---|---|---|
| 0 | >300 | Normal |
| 1 | >300 | Diffuse Allograft Infiltrates |
| 2 | 200–300 | Diffuse Allograft Infiltrates |
| 3 | <200 | Diffuse Allograft Infiltrates |
PGD severity is qualified by the extent of hypoxemia with reference to the administered FiO2 (PaO2/FiO2 ratio) (Table 1). LIRI and PGD are associated with adverse outcomes including prolonged mechanical ventilation, increased post-operative length of stay, and increased mortality [7][29][30]. The presence of stage 3 PGD within 72 h is strongly associated with poor outcomes [31]. This is in part related to the systemic consequences of LIRI that reach beyond the lungs and result in multisystem organ dysfunction.
At present, there is no specific treatment for the consequences of LIRI and PGD, only supportive therapy to allow time for recovery and limit secondary damage. Paradoxically, reperfusion of the ischemic organ is the required treatment, which simultaneously triggers the cascade of events that can lead to organ compromise. Supportive measures include the use of protective ventilation with permissive hypercapnia, moderate to high PEEP (8–10 cm H2O), low tidal volumes (6–8 mL/kg), and low peak inspiratory pressures (≤30 cm H2O) to avoid overdistension of alveoli [33]. Judicious fluid management to avoid fluid overload, and the optimization of hematocrit (25–30%) along with coagulation parameters are also targeted [34]. In cases resistant to standard supportive measures, inhaled nitric oxide can help lower pulmonary artery pressures and correct ventilation-perfusion mismatch [35]. Extracorporeal membrane oxygenation (ECMO) can also be employed as a final lifesaving measure. ECMO can help protect the lungs from aggressive ventilatory requirements for oxygenation goals while mitigating the harmful systemic effects of hypoxia [36][37]. Given the severity of repercussions from LIRI and PGD, it is important to further understand the pathophysiology to guide research, prevention, and treatment.
Research into preservation strategies to decrease the risk of PGD are needed and ex-situ lung perfusion is the foremost technological advancement in this field. Annually, rates of lung transplantation are increasing along with the average age of recipients [38]. Post-transplantation complications continue to be a major concern [39][40][41][42]. As previously mentioned, PGD due to LIRI has a reported incidence of 11-57% following LTx with a significant impact on survival outcomes [39][43]. PGD is also associated with increased short- and long-term morbidity, including the development of bronchiolitis obliterans syndrome (BOS) [5][30].
BOS is the clinical manifestation of underlying irreversible airway narrowing and is caused in part by LIRI and PGD [44]; therefore, reducing rates of LIRI and PGD by protecting the donor pulmonary and bronchial endothelium through ESLP may provide long-term clinical benefits. BOS is the leading cause of long-term morbidity, decreased quality of life, and mortality post-LTx [45][46]. BOS is most commonly caused by obliterans bronchiolitis, the progressive narrowing of the bronchioles via fibro-obliteration; however, other causes can produce the clinical effects of BOS, such as chronic rejection [46][47][48][49]. For this reason, chronic lung allograft dysfunction (CLAD) is a more encompassing term for post-transplant airway dysfunction, whereas BOS specifically refers to the most common cause of CLAD, which is obliterative bronchiolitis [50][51]. Clinically, BOS patients present with progressive worsening of lung function as measured by PFTs with a decrease in FEV1 measurements [51]. This syndrome develops in 30–50% of LTx patients within 3–5 years after surgery [52]. CLAD and BOS represent significant limitations to the enduring success of LTx. Treatment with high-dose steroids, ATGAM, and OKT3 therapy are largely unsuccessful at controlling its progression [46][53][54]. The precise mechanism of post-inflammatory fibrosis is not well established; however, studies of LIRI after LTx have demonstrated an increase in inflammatory mediators within the lung allograft [48][54], which may, in turn, upregulate the host immune response.
Technology that protects the pulmonary endothelium is essential to prevent these complications. ESLP of donor lungs has proven to decrease levels of inflammation, increase the donor pool, expand the geographic reach for transportation of organs, and recondition extended criteria lungs suitable for transplant [55][56][57][58][59][60][61][62][63][64]. ESLP also serves as a unique tool of investigation that allows for an improved understanding of the pathological processes of LTx.
Normothermic (37° Celsius) ESLP is a means of mechanically ventilating and perfusing donor lungs under physiologic conditions that improve organ quality and prolongs preservation compared to CSP [39]. The standard setup for ESLP involves the following steps. The donor lungs are intubated via the trachea and attached to a ventilator. The lungs are ventilated at a respiratory rate of approximately 10–20 breaths per minute with an FiO2 of 12–50%. The pulmonary trunk is cannulated and perfused with a solution to assist in preservation and reconditioning. The perfusate solution supports metabolism with varying compositions depending on the specific protocol being followed [56][64][65][66][67][68][69]. The perfusate drains from the left atrium into a circuit for filtration and deoxygenation before returning to the pulmonary artery to complete its course. The perfusate is propelled by a centrifugal pump, similar to cardiopulmonary bypass. The circuit includes an arterial line filter to capture debris and air bubbles (particulate filter). Some circuits include leukocyte filters. It also includes a membrane oxygenator (with a built-in heater-cooler) that functions as a deoxygenator with the addition of a standard sweep gas mixture (89% N2, 8% CO2, 3% CO2). The sweep gas replicates systemic oxygen demand.
The perfusate biochemistry is representative of the donor lung tissue function and allows for facile assessment of organ stability. Arterial blood gas samples are routinely assessed from the perfusate circuit, informing users of acid-base status, electrolyte concentrations, and quality of oxygenation/ventilation status. Where CSP is a black box, ESLP is an interactive, real-time evaluation and therapeutic tool. It greatly increases user control of donor organ quality during transportation and helps mitigate ischemic reperfusion injury.
Although CSP remains the gold standard method for organ preservation, ESLP is becoming the superior preservation strategy, and many researchers have contributed to improving its methodology. A noteworthy contribution was by Dr. Stig Steen and his team in 2001 when they described the first human transplantation of a donor lung reconditioned on ESLP [55]. This was particularly triumphant as the lungs came from a DCD donor—a person who has died from circulatory death as opposed to the neurologic determination of death (NDD/brain death). DCD lungs were previously shown to be viable for transplantation; however, there was a need to accurately assess them prior to transplantation, which was the benefit provided by ESLP. This clinical development spurred research in the field of ESLP as a means of expanding the organ donor pool by using DCD lungs. Since then, numerous studies have shown that normothermic ESLP produces less edema, superior alveolar-epithelial tight junction integrity, better metabolic function, and improved oxygenation compared to CSP [58][60][61][62].
ESLP has been shown to decrease the extent of LIRI and pulmonary inflammation compared to CSP. ESLP use in transplantation has resulted in a global increase in lung transplants worldwide [70]. Portable ESLP machines reduce cold ischemic times and perfuse/ventilate the lungs at normothermic temperatures during transport. The use of a buffered perfusate solution and pharmacological mediators of inflammation reduces the overall inflammatory insult on lungs incurred from retrieval. ESLP has been demonstrated to reduce the severity of inflammation in donor lungs post-ischemic reperfusion by decreasing allorecognition, infiltration, and priming of recipient T cells [71]. This in part explains why ESLP contributes to reconditioning of lungs and improved graft quality at transplantation.
There are two sources of blood supply in the lungs – the pulmonary and bronchial vessels and part of the benefit of ESLP may be due to the retrograde perfusion of the bronchial arteries with high oxygen content perfusate. The lungs are predominantly perfused by the pulmonary arteries that branch from the right ventricle. These arteries are low-pressure systems of hypoxic blood. The bronchial arteries branch off of the thoracic aorta (and occasionally the intercostal arteries and coronary arteries), supplying high pressure, high oxygen content blood to the bronchial walls and surroundings structures. Although the bronchial arteries only receive approximately 1% of the cardiac output due to their small diameter [72][73][74], these vessels serve an important function, and their absence can lead to the ischemic necrosis of airway mucosa. The two circuits coalesce in capillary beds (bronchopulmonary anastomoses) near the alveolar ducts [75]. The capillary beds drain into the pulmonary veins to join the left heart. The bronchopulmonary anastomoses allow for the mixing of systemic and pulmonic blood. This continuity also serves as a protective structure against ischemia in the event of an obstruction to flow in either circuit (flow through either system can help perfuse the other). The oxygen-rich alveoli and dual circulation of the lungs provide a greater degree of protection from ischemia compared to other solid organs [13]. Unlike other solid organ transplantation, LTx is unique in that it does not reestablish systemic circulation as the bronchial arteries are not re-anastomosed [76]. This is because the technique for bronchial artery revascularization has been unreliable clinically [77]. Therefore, much of the donor bronchus viability is the result of retrograde perfusion through the bronchopulmonary capillary beds by the pulmonary arteries [78]. During CSP, the pulmonary arteries are likely more resistant to the ischemia due to their typically hypoxic circulation, whereas bronchial arteries are systemic vessels, used to systemic pressures and high oxygen contents and may be more severely affected. ESLP provides retrograde perfusion of the bronchial arteries and their surrounding structures via highly oxygenated flow through the pulmonary vessels. This protective situation may account for some of the improved graft performance and decreased inflammation seen in lungs managed by ESLP compared to CSP.
NPV ESLP has been shown to produce improved outcomes compared to PPV ESLP in animal models [64]. There are two ventilation strategies employed in commercially available ESLP platforms: negative pressure ventilation and positive pressure ventilation. The Ex-vivo Organ Support System (EVOSS) is the only platform for humans that currently uses negative-pressure ventilation (Figure 1). This means that the EVOSS more closely replicates physiologic respiration—ventilation is achieved by pulling the lungs open with an extrapleural vacuum rather than forcing the lungs to expand by exclusively inflating them with air. In 2018, Aboelnazar et al. demonstrated that NPV-ESLP is associated with reduced inflammation and lung injury compared to PPV, irrespective of use with cellular or acellular perfusate [64]. The team also determined that human lungs lost weight during the NPV run with cellular perfusate, suggesting a “drying out effect” with this combination of ESLP strategy. Where edema is that hallmark of LIRI, the associated decrease in inflammatory markers and weight gain following NPV suggests its superior ability at mitigating LIRI.
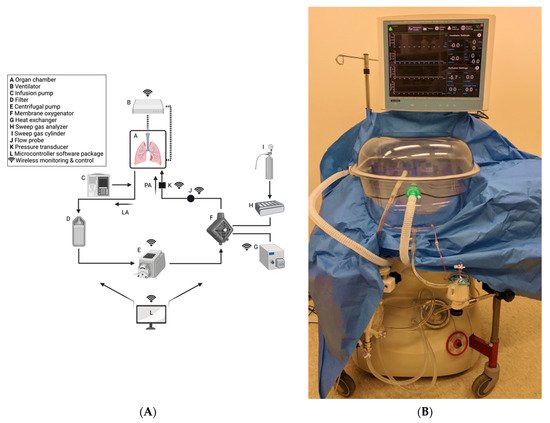
Figure 1. Custom-built NPV-ESLP platform: (A) Schematic of NPV-ESLP components; (B) Tevosol Inc. clinical trial prototype. (A) Oxygenated perfusate drains from the open left atrial system into a hard-shell reservoir (A), then through an arterial/particulate filter (D), pumped via the centrifugal pump (E) to the oxygenator/heat exchanger (F,G); which in turn warms the perfusate to normothermia (computer-controlled/adjusted (L)) and deoxygenates it with a sweep gas mixture (H,I). Prior to re-entering the lungs via the pulmonary arterial cannula (PA) for re-oxygenation, the perfusate passes by a flow probe sensor (J) and a pressure transducer (K). Negative Pressure Ventilation (NPV) circuit is depicted (B). An infusion pump (C) infuses insulin, dextrose, volume, and medications as needed.
EVOSS was developed by Dr. Darren Freed and Dr. Jayan Nagendran in 2016 at the University of Alberta in Edmonton, Canada. The aim was to develop a form of ESLP that reduced the incidence of ventilator-induced lung injury (VILI)—a mechanically induced pulmonary edema caused by high respiratory pressures and a documented consequence of prolonged PPV-ESLP [79][80][81]. A recent clinical trial demonstrated that NPV is safe when used to recondition extended criteria donor lungs from transplantation [82].
In 2020, Buchko et al. established the clinical safety and feasibility of EVOSS technology through a single-center clinical trial of NPV-ESLP whereby twelve extended criteria donor lungs were successfully reconditioned and transplanted into recipients, resulting in 100% 30-day and 1-year survival [82]. Recruitment took place between October 2018 to July 2019. Extended criteria donors were procured in the standard fashion with CSP during transportation to the implanting hospital. Lungs were then connected to EVOSS for preservation, reconditioning, and evaluation. If the lungs were deemed acceptable for transplantation, they remained on the NPV-ESLP until the first recipient lung was explanted. The average ESLP run was 182 min, and total cold ischemic time was approximately 308 min and 359 min for the right and left lungs, respectively. The average total time from donor explant to implantation was 8 h 14 min and 9 h 6 min for right and left lung, respectively. The mean P:F ratio was 492 and all organs met the criteria for utilization, including stable hemodynamics and oxygenation after 3 h of NPV-ESLP. In addition to the excellent survival outcomes from this trial, no patients developed PGD scores grade 3 at 72 h or required extracorporeal membrane oxygenation (ECMO) post-operatively. This study demonstrates very promising results for the commercial use of EVOSS. To further establish the clinical utility of this technology a multi-center clinical trial is required, which will be the horizon for the EVOSS team.
ESLP also provides a means of delivering targeted therapies to the donor lungs, which has multiple advantages. First, the treatments can be delivered to the lungs in isolation, which avoids any potential negative impact on other organ systems and promotes a concentrated treatment exclusive to the lungs. Second, the continual evaluation of the donor lung function on ESLP enables real-time assessment of cause-and-effect. Third, because the lungs are treated in isolation, the effects seen in animal models can more easily be translated into clinical trials [1]. At present, gene therapies, stem cell therapies, receptor agonists, and inhaled agents are being used as specific ESLP treatments for animal models of lung transplantation.
ESLP allows for the delivery of targeted gene therapies using viral vectors. For example, inflammation has been reduced in donor lungs following treatment with IL-10 gene therapy via ESLP in large animal transplant survival models and nontransplant, damaged human lung models to achieve transplant acceptable parameters [83][84][85]. In another large animal model of gene therapy via ESLP, adenoviral IL-10 decreased evidence of allograft rejection and was associated with improved lung function when compared to controls or CSP alone [83].
Mesenchymal stem cells (MSC) delivery via ESLP is being studied for its therapeutic potential to reduce the signs of LIRI. Stone et al. (2017) have shown in a DCD murine model that MSC delivered to donor lungs via EVLP demonstrate improved edema and lower levels of neutrophil infiltration compared to controls. They also examined the BAL fluid and observed decreased inflammatory markers IL-17, TNF-α, CXCL1, and HMBG1 levels [86].
Selective adenosine 2A receptor (A2AR) agonists have been shown to improve lung parameters in transplantation models when administered via ESLP. In 2015, Stone et al. investigated the role of adding A2AR to ESLP perfusate in a murine model of lung donation compared to ESLP alone. Their findings demonstrated lower levels of inflammatory markers, including CXCL1, CCL2, and TNF-α, decreased concentration of neutrophils, lower PA pressures, and improved lung compliance [87].
Certain inhaled agents have shown beneficial results on lung function when used in conjunction with ESLP. For example, sevoflurane, a volatile anesthetic, has been shown to provide significant protection from LIRI in a rat model of DCD lung donation with reconditioning by ESLP. Wang et al. (2018) found that administering 2% sevoflurane over 3 h of ESLP resulted in reduced inflammation with lower levels of TNF-α along with less weight gain and perivascular edema compared to ESLP without sevoflurane [88]. In similar studies of canine ESLP, β-adrenoreceptor agonists administered during ESLP have demonstrated improved lung function as assessed by increased PaO2 and compliance [89][90]. These findings suggest that sevoflurane and β-adrenoreceptor agonists have the potential to protect lung function and can help recondition damaged lungs prior to transplantation.
As outlined above, ESLP is the way forward to improve lung transplant outcomes: increase the number of transplantations performed each year, expand the geographic distance over which donor lungs can be retrieved, and provide a vehicle to deliver therapeutics. ESLP has proven instrumental for research into the cause and treatment of LIRI in clinical and laboratory settings [91][92][93]. For patients, it has improved donor lung availability and quality, which translates into more lives saved.