1000/1000
Hot
Most Recent

Osteoclasts are derived from hemopoietic progenitors of the monocyte-macrophage lineage. They differentiate upon exposure to macrophage colony stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL), which are presented by osteoblasts and osteocytesare. Multinucleation in a late phase of osteoclastogenesis is a hallmark of osteoclast maturation. The unique and dynamic multinucleation process not only increases cell size but causes functional alterations through reconstruction of the cytoskeleton, creating the actin ring and ruffled border that enable efficient bone resorption.
The process of osteoclast multinucleation is dynamic, complicated and finely controlled by multiple entangled factors. At the beginning of the 21st century, two master fusogens, DC-STAMP and OC-STAMP, had been identified that directly regulate osteoclast multinucleation.
The identification of DC-STAMP and OC-STAMP, two “master fusogens” in osteoclasts, was a breakthrough in the first decade of the 21st century [1][2]. As the direct ligands for these two cell-membrane receptors have not been identified, however, the investigation of their downstream signaling pathways has been difficult. Despite the identification of several other molecules regulating osteoclast multinucleation, such as ATP6v0d2, CD47-MFR, ITAM-bearing immunoreceptors, the overall view of the multinucleation mechanism remains obscure.
DC-STAMP was first identified through a random cDNA library sequencing of human monocyte-derived dendritic cells. This 470-amino-acid protein is expressed at the cell surface [3] and on the endoplasmic reticulum (ER) membrane [4][5], and contains seven putative transmembrane regions with an intracellular C terminus. TRAP-positive mononuclear osteoclasts were observed in DC-STAMP-/- mice, and their expression levels of osteoclast markers and transcription factors such as c-Fos, NFATc1 and Cathepsin-K were comparable to those in wild-type mice, yet their osteoclast multinucleation was completely abrogated.
There is widespread agreement that DC-STAMP works through receptor-ligand interaction. To date, however, the identity of DC-STAMP’s ligand remains unclear. The cytoplasmic tail of DC-STAMP contains several serine residues, two of which are considered targets for phosphorylation. The details of the intracellular reactions are still largely unknown, however.
DC-STAMP is regulated by various entangled molecules. RANKL-RANK is an important initiator of the expression of DC-STAMP via MAPK cascade, AP-1 and NFATc1. These two master transcription factors (AP-1 and NFATc1) and other factors including MITF, PU.1, Bcl6, and Blimp6 work together in a coordinated manner to control DC-STAMP with precision. Micro RNAs and HDACs also exhibit various effects on the regulation of DC-STAMP. Once the mRNA of DC-STAMP is translated into protein, it binds to LUMAN and OS9 for ER-to-Golgi transportation and eventually reaches the plasma membrane. Signaling through the ITIM that exists at the intracellular tail of DC-STAMP, conversely, regulates AP-1 and NFATc1 in a positive direction, further enhancing osteoclast differentiation and multinucleation.
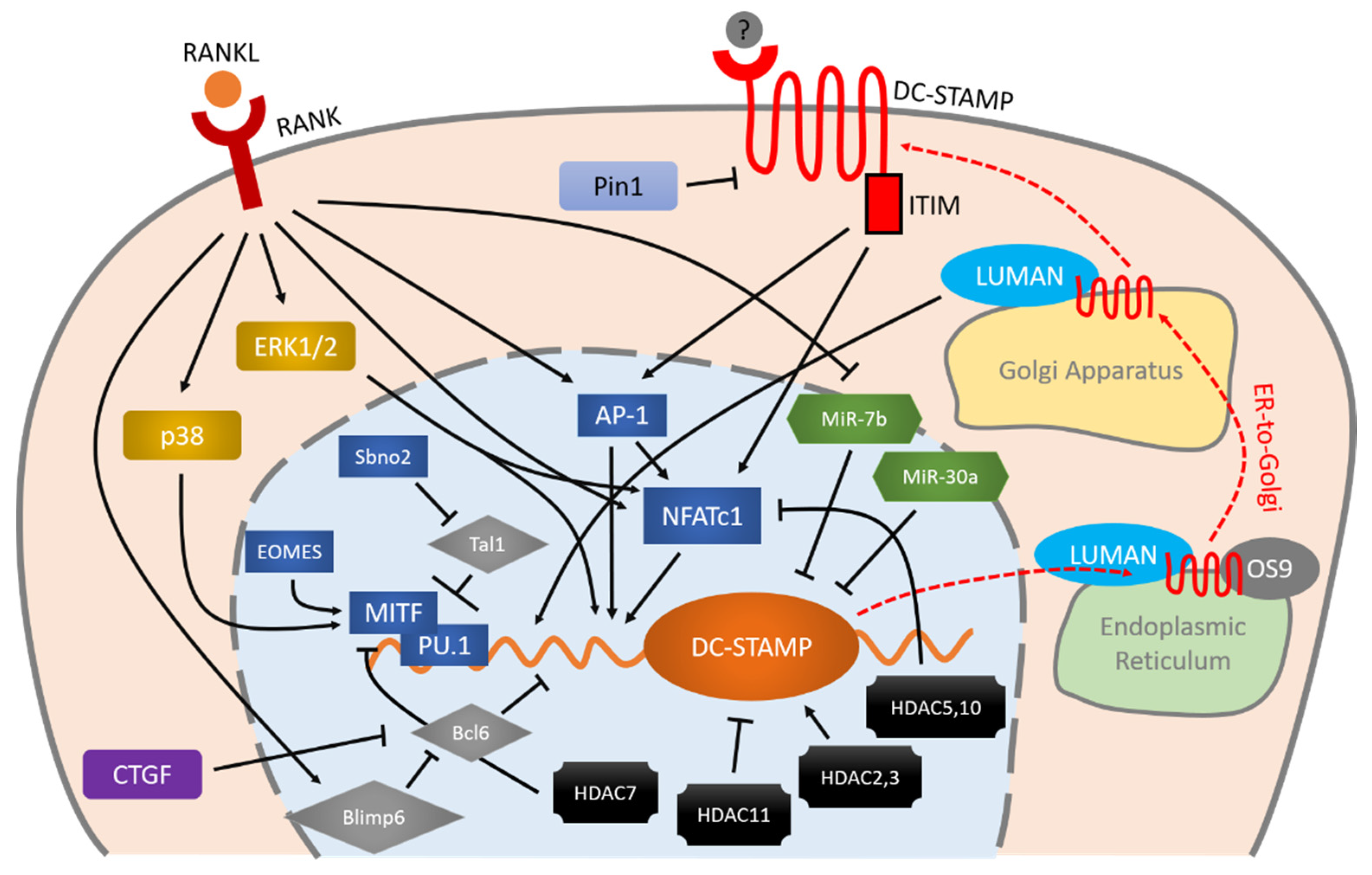
Figure 1. Schematic illustration of DC-STAMP regulation during osteoclastogenesis. RANKL-RANK is an important initiator of the expression of DC-STAMP via MAPK cascade, AP-1 and NFATc1. These two master transcription factors (AP-1 and NFATc1) and other factors including MITF, PU.1, Bcl6, and Blimp6 work together in a coordinated manner to control DC-STAMP with precision. Micro RNAs and HDACs also exhibit various effects on the regulation of DC-STAMP. Once the mRNA of DC-STAMP is translated into protein, it binds to LUMAN and OS9 for ER-to-Golgi transportation and eventually reaches the plasma membrane. Signaling through the ITIM that exists at the intracellular tail of DC-STAMP, conversely, regulates AP-1 and NFATc1 in a positive direction, further enhancing osteoclast differentiation and multinucleation. Arrows indicate positive regulation, bars indicate negative regulation, and red dashed arrows indicate intracellular transportation of DC-STAMP.
Paul R. Odgren’s group was the first to identify OC-STAMP using a microarray analysis of mouse primary bone marrow cells and RAW264.7 cells with/without RANKL stimulation. The gene encoding OC-STAMP is located on chromosome 2H3, and is strongly conserved in mammals including mice, rats and humans [1]. Odgren’s group later analyzed the protein structure in detail, revealing that OC-STAMP’s protein structure is similar but not identical to that of DC-STAMP. It penetrates the plasma membrane six times, and both the N- and C-termini are intracellular tails. There is a glycosylation site in the extracellular region that contributes to protein stability [6]. Initially, the expressions of OC-STAMP and DC-STAMP were confirmed in organs where cell fusion occurs, such as bone, muscle and skin [7]. Later, their universal expression was demonstrated in almost all human organs. Notably, the expression of OC-STAMP is much lower in the ovary than in other organs, and later experiments have suggested that estrogen influences its expression [8].
Osteoclast multinucleation is completely inhibited in OC-STAMP-deficient mice, which nevertheless show normal growth and skeletal systems [6][7]. OC-STAMP-deficient cells isolated from bone marrow were able to differentiate into TRAP-positive osteoclasts under RANKL stimulation but could not fuse into multinucleated cells. The bone resorption ability of these mononuclear cells was about one-third that of wild-type cells. The expression levels of other osteoclast markers (Cathepsin K, NFATc1, TRAP, DC-STAMP) were not significantly altered, indicating OC-STAMP’s specific role in osteoclast multinucleation rather than osteoclast differentiation.
Little is known about the regulation of OC-STAMP. The fact that OC-STAMP expression in bone marrow monocytes increases only after stimulation with M-CSF and RANKL indicates that OC-STAMP is a RANKL-induced protein [8][9]. To date, however, specific knowledge about the regulation of OC-STAMP remains limited. NFATc1 is the master transcription factor of OC-STAMP, but Akt, NF-κB and PKCβ also play an important role in OC-STAMP’s regulation which is independent of NFATc1. OC-STAMP-induced signaling may positively influence other fusogens such as CD9 and meltrin-α, enabling them to regulate osteoclast multinucleation together.
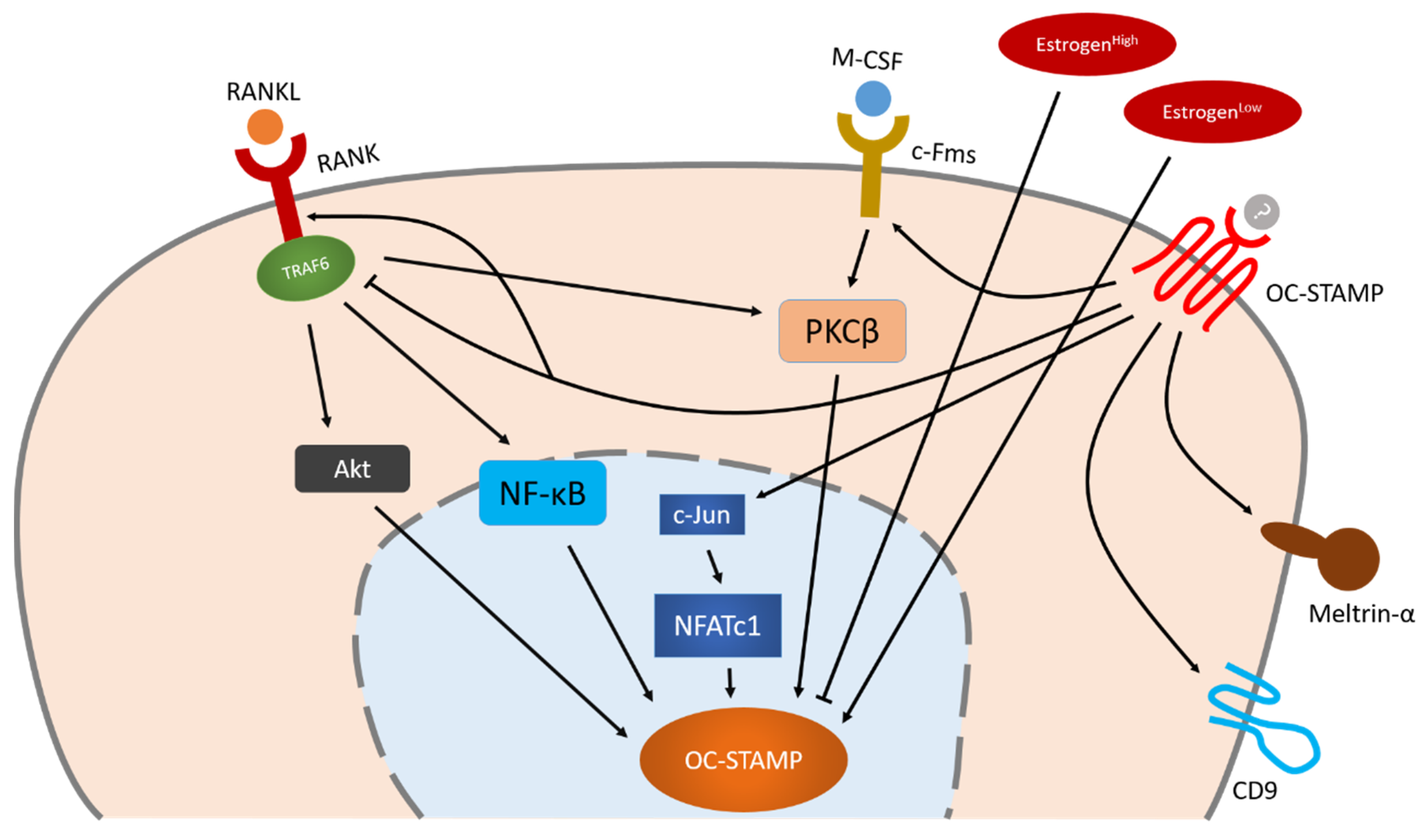
Figure 2. Schematic illustration of OC-STAMP regulation during osteoclastogenesis. NFATc1 is the master transcription factor of OC-STAMP, but Akt, NF-κB and PKCβ also play an important role in OC-STAMP’s regulation which is independent of NFATc1. OC-STAMP-induced signaling may positively influence other fusogens such as CD9 and meltrin-α, enabling them to regulate osteoclast multinucleation together. Arrow indicate positive regulation, bars indicate negative regulation.
Other than the two STAMPs, recently discovered molecules such as those on the siglec-15/DAP12 axis and the NETRIN-1/Flrt2/Unc5b axis as well as the purinergic receptors have been found to regulate osteoclast multinucleation as well. Despite the constant efforts of researchers to understand the mechanisms controlling osteoclast multinucleation from various angles, the process remains, to a large extent, unexplained.
The process of osteoclast multinucleation is dynamic, complicated and finely controlled by multiple entangled factors. Multinucleation is considered to be the result of osteoclast fusion, but multiple fusion patterns have been observed between different fusion partners, depending on their cell morphology, i.e., whether they are mononuclear or multinuclear, and their stage of osteoclastogenesis [10]. Moreover, as Takegahara et al. have recently demonstrated, incomplete cytokinesis of osteoclasts can also cause multinucleation [11]; this finding offers us a completely new angle from which to investigate the process.
The fact that we have not yet identified the direct trigger for osteoclast multinucleation is hindering our understanding of the overall process. RANKL can induce osteoclastogenesis and subsequent multinucleation but is not the direct regulator of multinucleation. RANKL-unstimulated monocytes have been observed to fuse with RANKL-stimulated osteoclast progenitors [12], proving that RANKL signaling only indirectly induces osteoclast fusion. Deficiency of DC-STAMP and/or OC-STAMP completely abrogates osteoclast multinucleation but not osteoclast differentiation under RANKL stimulation, indicating that these two molecules act as “master fusogens” in osteoclasts, but their ligands are unknown. Researchers have developed innovative methods of directly triggering signals in osteoclast multinucleation; Verma et al., for example, have used LPC (lysophosphatidylcholine) to pause osteoclasts in a pre-fusion state in order to synchronize their fusion processes [13][14]. Chiu et al. engineered a DC-STAMP chimeric molecule in which light exposure mimics its ligation to explore its downstream signals [15]. The side effects of these interventions on osteoclasts remain unknown, however. There is an urgent need for the identification of the original physiological molecules that directly trigger osteoclast multinucleation.