Cancer stem cell-targeted therapies means therapies targeted at cancer stem cells.
1. Introduction
Targeting stromal cells has become one of the strategies to destem CSCs. Many preclinical and clinical trials are being conducted to evaluate the therapeutic efficacies of various small molecule inhibitors/neutralizing antibodies in disrupting the interactions between stromal cells and cancer cells. The current direction in this field is to dissect how stromal cells recruit and regulate various immune suppressive cells to create an immunosuppressive TME. Single-cell RNA sequencing analysis provides a dynamic stromal niche that supports cancer stemness and immune evasion in various cancer types. Strikingly, this technique will provide mechanistic insight and a novel strategy for current immune checkpoint therapy. Traditionally, CSCs were regarded as subpopulations within the tumor that promote tumor recurrence and therapeutic resistance. Accumulating evidence has demonstrated the distinct role of CSCs in immune evasion. Immense effort has currently been made to dissect the crosstalk between CSCs and various immune suppressive cells, including MDSCs and Tregs. In addition, increasing interest is directed towards understanding the direct interaction between CSCs and macrophages and CD8+ T cells. Based on these interesting findings, CSC-targeted immunotherapy may be a promising approach for cancer therapy, and its therapeutic efficacy needs further investigation.
2. Interplay of Various Cellular Factors within the TME in the Regulation of Cancer Stemness and Immune Evasion
In the previous sections, we discussed how individual stromal cells and immune cells interact with CSCs within the TME. However, stromal cells do not regulate the plasticity of CSCs in isolation and have a highly context-dependent mechanism of action. As one of the major stromal cells in the TME, CAFs interact with various stromal cells and immune cells in the TME to regulate CSC plasticity and immune evasion. First, a number of reports have demonstrated the crosstalk between CAFs and macrophages in the promotion of cancer stemness. CAFs play a crucial role in the promotion of cancer stemness in HCC by reciprocally inducing the activity of TAMs
[1]. Overexpression of these markers, including α-SMA and CD68
+, is associated with HCC recurrence and shorter overall survival
[1]. Recently, Yang et al. showed that CAFs express endosialin, which regulates macrophage recruitment and polarization to support HCC progression
[2]. In prostate cancer (PCa), Comito et al. identified crosstalk among different cellular components, including CAFs, TAMs and PCa cells, leading to the promotion of cancer stemness
[3].
Apart from TAMs, CAFs also interact with endothelial cells in the TME. CAFs regulate the endothelial lipoma-preferred partner (LPP) gene in endothelial cells, rendering a chemoresistant phenotype in ovarian cancer
[4]. Moreover, Song et al. reported novel cytokine-mediated crosstalk among CAFs, HCC cells and TANs, augmenting cancer stemness and TAN recruitment in HCC
[5]. Specifically, CAF-derived CLCF1 recruited and promoted N2 polarization of TANs via induced secretion of CXCL6 and TGF-β in HCC cells
[5]. Accumulating evidence has also demonstrated the interaction between CAFs and immune cells in promoting an immunosuppressive environment. CAFs promoted recruitment of CCR2+ monocytes and conversion to the MDSC phenotype via preferential secretion of CCL2, which created an immunosuppressive environment
[6]. Targeting the CAF-MDSC axis by CCR2 inhibition may open a promising therapeutic avenue for converting from a non-T-cell-inflamed TME to a T-cell-inflamed counterpart in lung carcinoma
[6]. In addition to MDSCs, the CAF-neutrophil axis was reported to be a promising approach for the development of stromal treatments in pancreatic cancer. CAF-derived CXCL12 recruited neutrophil infiltration, which led to resistance to T-cell mediated killing
[7]. In addition, CAFs recruit and enrich the Treg population via IL6 secretion in esophageal cancer
[8]. Last, CD73
+ CAFs suppressed T cell activity in a colon cancer model via A
2A-mediated immune suppression, and thus targeting the CD73-adenosine pathway is a promising approach for complementing PD1 therapy
[9].
3. Conclusions
The dynamic interactions between stromal cells and CSCs are not simply unidirectional but reciprocal, promoting the expansion of stem cell markers, migration, invasion, drug resistance and self-renewal properties of CSCs (Figure 1).
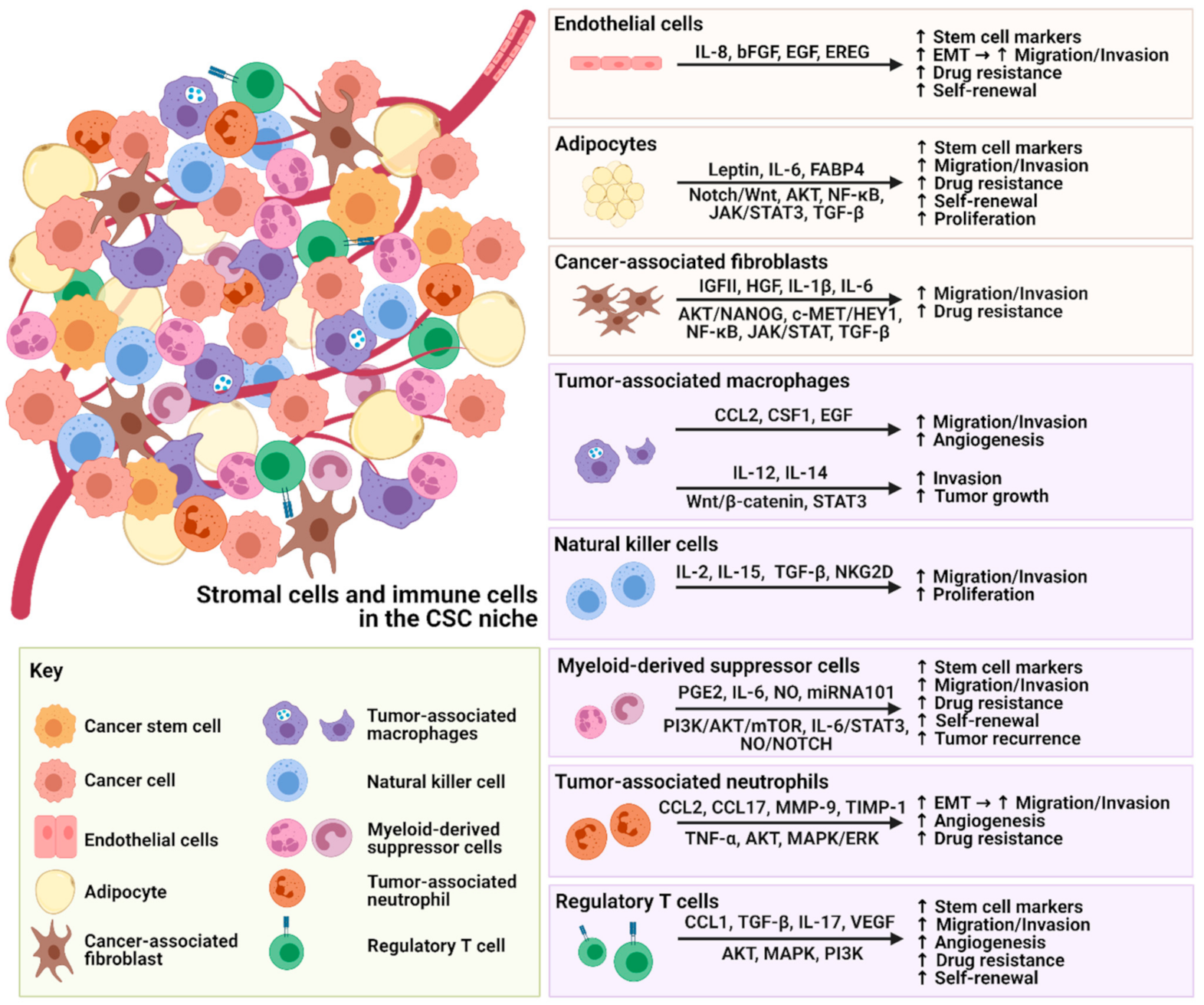 Figure 1.
Figure 1. Illustration of the crosstalk between stromal cells, immune cells and CSCs.
Similar tumor-promoting crosstalk is also observed with immune cells, leading to immune evasion of CSCs and tumor recurrence. An increasing number of studies have focused on targeting the major secretomes or molecules related to stromal cells and immune cells in the TME, which are summarized in Table 1. These therapeutic inhibitors or neutralizing antibodies could potentially be used as single treatments or in combination with current therapies for better treatment outcomes. However, it is not yet clear whether these promising preclinical strategies that enhance our understanding of cancer development will translate into effective treatment for cancers. More knowledge needs to be gained, and convincing preclinical results need to be evaluated in clinical trials—there is still a long way to go. Although these targets have been reported, their effects on cancer phenotypes may be tumor-type specific. Some solid tumors and liquid tumors may have different responses to stromal cells and immunotherapy. Therefore, hampering stromal cells as a therapeutic strategy to destem CSCs needs further investigation.
Table 1. Therapeutic strategies targeting the interactions between CSCs and stromal cells for cancer treatments.
| Type of Targeted Cell |
Cancer Type |
Novel Therapeutic Strategies |
Mode of Action |
Effects on Cancer Stemness |
References |
| Targeted Stromal Cells |
- |
| Endothelial cells |
Glioblastoma |
IL-8 neutralizing antibody |
Blockade of endothelial cell-secreted IL-8 |
Reduced spheroid size and tumor growth in vivo |
[10] |
| Adipocytes |
Breast/Mammary cancer |
BMS309403 |
Inhibiting FABP4 functions |
Suppressed tumor growth and tumor volume with decreased IL-6 level and tumor ALDH1 activity |
[11] |
| Ovarian cancer |
Anti-OB-R blocking peptide |
Blockade of leptin receptor |
Decreased leptin-induced cell migration and invasion abilities |
[12] |
| CAFs |
Breast Cancer |
GW4064 |
Agonist of FXR |
Reduced progression and motility of tumors |
[13][14] |
| Pirfenidone (PFD) and doxorubicin |
Inhibiting collagen production and tumor growth |
Reduced components of ECM, inhibited tumor growth and lung metastasis |
[15] |
| Targeted Immune Cells |
- |
| TAMs |
Pancreatic carcinoma |
CD40 Agonists |
Activating and inducing macrophages |
Degraded ECM and improved tumor infiltration of immune cells |
[16] |
| Non-small cell lung cancer (NSCLC) |
IL-33 neutralizing antibody |
Blockade of IL-33 |
Inhibited M2-like macrophages polarization via inhibition of IL-10 and VEGF as well as reduced accumulation of Treg cells |
[17] |
| NK cells |
Colon cancer |
Chondrocytes |
Expressing a high level of IL-12 |
Increased the infiltrations of both T cells and NK cells, reduced cancer cells and tumor angiogenesis |
[18] |
| MDSCs |
Breast cancer |
Combination of anti-IL-6 antibody and iNOS inhibitor |
Targeting MDSC-derived IL-6 and nitric oxide |
Reduced spheroid formation stimulated by MDSCs |
[19] |
| Endometrial cancer |
Doxorubicin and Gr-1 neutralizing antibody or celecoxib |
Blockade of MDSCs or inhibiting MDSC functions |
Reduced ALHD+ expression and sensitized tumor cells to chemotherapy |
[20] |
| TANs |
Breast cancer |
Zileuton |
Inhibiting neutrophil Alox5 |
Reduced lung metastasis |
[21] |
| Treg cells |
Glioma |
Tocilizumab |
Blockade of IL-6 receptor |
Inhibited tumor growth and CD133 expression induced by Treg cells |
[22] |
| Acute myeloid leukemia (AML) |
IL-10R neutralizing antibody |
Neutralizing IL-10 receptor functions |
Reduced side population, sphere formation ability, and expression of OCT4 and NANOG |
[23] |
| Ovarian cancer |
CD25 neutralizing antibody |
Inhibiting CD25+ Treg cells |
Reduced angiogenesis and inhibited tumor growth |
[24] |

 Figure 1. Illustration of the crosstalk between stromal cells, immune cells and CSCs.
Figure 1. Illustration of the crosstalk between stromal cells, immune cells and CSCs.