1000/1000
Hot
Most Recent

Cardiac imaging techniques include a variety of distinct applications with which we can visualize cardiac function non-invasively. Through different applications of physical entities such as sound waves, X-rays, magnetic fields, and nuclear energy, along with highly sophisticated computer hardware and software, it is now possible to reconstruct the dynamic aspect of cardiac function in many forms, from static images to high-definition videos and real-time three-dimensional projections.
Even during the perilous era of COVID-19, the burden of cardiovascular disease in the modern world is quite severe. This occurs not only because CVD represents one of the single highest causes of mortality in high-income countries, but mostly because it is in the top five most common causes of mortality in low-income countries with a growing tendency in the years to come [1]. Thus, “seeing” and understanding cardiac function is imperative, first to help us differentiate normal and healthy from abnormal and pathological, and second to guide the physicians towards an appropriate treatment for the patients that are being affected by cardiovascular disease.
Cardiac imaging consists of many different modalities that can reproduce a visual representation, meaning images and videos from which, after proper analysis, we can extract useful information concerning cardiac function. This general term of “cardiac function” describes a sequence of precisely timed electromechanical events such as chamber contraction, blood outflow, chamber expansion, blood inflow, valvular openings and closures. This sequence makes the heart work as a hydraulic pump that supplies oxygenated blood to the rest of the body.
Myocardial perfusion imaging (SPECT) is a well-established method to evaluate cardiac function. More precisely it can access coronary artery function and can accurately differentiate regions of viable from ischemic, stunned, or scarred myocardium. It involves the injection of intravenous radioactive tracers that are trapped in the myocytes after crossing throughout the vasculature surrounding the myocardium. The commonly used isotopes for these studies are either Thallium-201 or Technetium-99m. These radioactive tracers emit radioactive energy that can be detected, absorbed, and converted into perfusion images of the human heart [2][3][4].
Positron Emission Tomography (PET) has multiple clinical and research applications in CV imaging. It offers accurate measurements of global and regional wall motion, giving insight to myocardial perfusion, and myocardial blood flow during rest and stress, all in one exam. Myocardial viability is routinely estimated by quantitative comparison of fluorodeoxyglucose ( 18FDG) absorption between rest and stress. Myocardial blood flow and coronary flow reserve measurements are also included in the final clinical assessment due to the enhanced dynamic imaging capacities of the latest PET/CT scanners, which is considered the “gold” standard technique. Absolute flow measurements also allow evaluation of coronary microvascular dysfunction and provide additional prognostic and diagnostic information for coronary artery disease. Standard quantitative approaches to computing myocardial blood flow from kinetic PET data in an automated and rapid fashion have been developed for 15O-water, 13N-ammonia, and 82Rb radiotracers. Computerized analysis of perfusion in comparison to visual analysis reduces examination variability. PET quantification can also be enhanced by precise co-registration with CT angiography. In emerging clinical utilities, PET has demonstrated a prospective imaging method capable of identifying vulnerable atherosclerotic plaques by quantification of plaque uptake of 18FDG and 18F-sodium fluoride tracers, not only in large arteries such as the carotids and aorta, but also in smaller arteries such as the coronary and renal arteries [5][6][7][8][9][10].
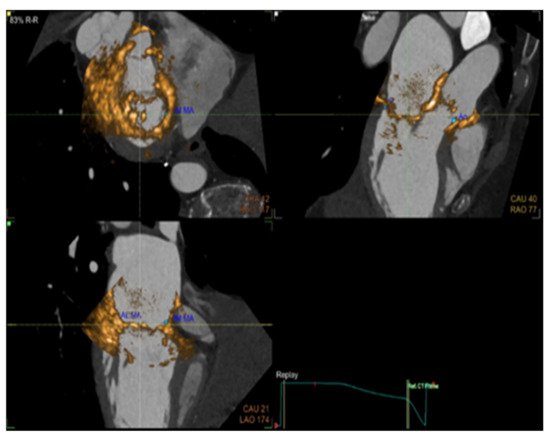
The evaluation of cardiac function in real-time with increased spatial resolution has become critically important during transcatheter interventions and is gaining ground day by day as a mainstay in cardiac care with regard to treatments for structural heart disease. These procedures, while minimally invasive for patients, often have added degrees of patient-related and procedure-related complexity. Fluoroscopy has long been the cornerstone of interventional procedures due to the excellent device visualization and due to the fact that it provides real-time feedback. On the contrary, fluoroscopy offers poor characterization of non-radiopaque structures and provides only 2-D projections of important 3-D cardiac anatomy, lacking essential spatial resolution. Fusion imaging combines data from different imaging modalities, fluoroscopy, computed tomography, and echocardiography, in order to produce a fused image, video, or live streaming (Figure 1). With these methods, we have access to high-resolution detailed anatomic information from CT imaging combined with functional, real-time motion and flux information from echocardiography. The fusion of the pre-operative CT and the peri-procedural 4D-TEE provides us with an excellent visualization of both images in the same visual perspective on the echo screen in the catheterization laboratory [11][12][13][14][15].
Even though cardiac imaging is considered non-invasive, it is crucial to mention that during interventional procedures such as coronary interventions, new imaging modalities such as intravascular ultrasound (IVUS) and optical computed tomography (OCT) are currently deployed both clinically and experimentally with an established recognition, as has been demonstrated in recent guidelines [16]. Other techniques like optoacoustic (OA) imaging, also known as photoacoustic imaging (PAI), can outperform conventional imaging techniques in terms of temporal resolution, as shown in several research studies [17][18].
The field of cardiovascular imaging is about to be shifted by a huge wave of new methods concerning computational analysis of big data sets. Artificial intelligence (AI), machine learning (ML), deep learning (DL), and natural language processing (NLP) are forming a new paradigm for imaging analysis, with many emerging applications in almost every imaging technique. The development of AI applications with big imaging registries will likely facilitate and ameliorate the extraction of both functional and anatomical information and will offer more precise diagnostic tools in cardiovascular care [19].