1000/1000
Hot
Most Recent

Vascular regeneration remains a challenging issue in tissue engineering. Poor vascularization often limits the size of regenerated tissue and leads to cell death. Improving vascularization could largely increase the regeneration efficacy and the survival of regenerated tissue. Nanofibrous scaffolds are widely used in tissue engineering and regenerative medicine due to well-known advantages, which can mimic the mechanical and structural properties of the natural extracellular matrix (ECM). This review presents the recent strategies to improve nanofibrous scaffolds for vascular tissue engineering. Different nanofibrous scaffolds design, including nanofiber structuring and surface functionalization, to improve scaffolds properties are explained. The review also focuses on recent advances in electrospun fibrous scaffolds for vascular regeneration such as changing architecture and controlling the release of bioactive components. In vivo tests of these nanofibrous scaffolds have been considered as the further step to validate the angiogenic potential of the scaffolds.
Vascular regeneration is one of the most challenging issues in tissue engineering [1][2]. During formation of new tissue, blood vessels are required to supply oxygen and nutrition for cells, and remove waste products [3][4][5]. Lack of efficient vascularization limits the size of tissue-engineered constructs [6][7]. Implantation of tissue constructs in a poorly vascularized site often leads to lack of tissue integration and cell death [8][9]. As a result, many tissue-engineered constructs have been reported to fail in vivo due to the lack of vascular network formation [7][10][11]. Therefore, vascular regeneration is critical for the successful regeneration of tissues where vascularization is necessary [12][13]. Electrospinning is a commonly used technique to produce fibers at the micro/nano scale, which could mimic the mechanical properties of native ECM. The potential in vivo use of implantable electrospun tubular scaffolds for vascular graft was widely reported [14][15][16]. In particular, tubular electrospun scaffolds collected on a rotating mandrel showed advantages when mimicking the scale and architecture of vessels.
Nanotopography has been noted as an important factor with which to affect cell growth and differentiation. ECs could interact with their physical environments and be guided by a scaffold’s topography. Xu et al. reported that the alignment of nanofibers plays a positive role in the regulation of endothelial cellular behavior [17]. Cell elongation and migration are indispensable processes in angiogenesis. This study proved that the alignment of nanofibers could guide cell distribution, affect cell morphology, and even control migration velocity [18]. When HUVECs were cultured on PLGA-aligned nanofibrous scaffolds, the morphologies of migrating cells were highly ordered. Therefore, the topographic features and scaffold guidance should be evaluated when designing a tissue-engineered scaffold for vascular regeneration.
Apart from electrospun scaffolds, a few other studies showed that EC morphogenesis into capillary-like structures was regulated by micropatterned stripe substrates [19]. Dike et al. showed that ECs cultured on substrates micropatterned with 10 µm wide lines of fibronectin formed capillary tube-like structures containing a central lumen; cells cultured on wider (30 µm) lines did not form tubes [19]. Moon et al. micropatterned poly (ethylene glycol) diacrylate hydrogels with RGDS in different geometries [13]. As a result, ECs cultured on RGDS patterns reorganized their cell bodies into tube-like structures on 50 µm wide stripes, but not on wider stripes. These results suggested that EC morphogenesis could be regulated by topography cues. The development of a well-designed topography, in which capillary tubes consistently form, is an important step toward the fabrication of engineered tissues.
Many researchers have reported that the controlled release of angiogenic factors could promote vascular formation in vitro and in vivo. Electrospun nanofibrous scaffolds show potential to incorporate or functionalize bioactive components onto the scaffolds, and scaffolds designed for drug release have shown controlled delivery. VEGF is the most important growth factor to stimulate early vascular formation and promote angiogenesis. Jia and his colleagues loaded VEGF into the inner of core/shell fibrous scaffold by coaxial electrospinning with PLGA as the shell [20]. VEGF release could be sustained for more than 28 days and cell studies showed that VEGF encapsulated scaffolds effectively enhanced cell proliferation and benefited cell distribution. Another study demonstrated VEGF functionalized heparin-conjugated PCL fibrous scaffolds were able to release the growth factor for 15 days [21]. This resulted in new blood vessel formation with minimum immunological rejection. Other growth factors, such as bFGF [22] and PDGF [23], were also reported to be released from nanofibrous scaffolds for vascular regeneration. Montero et al. prepared bFGF-loaded gelatin fibrous scaffolds by physical adsorption after electrospinning [24]. HUVECs were seeded on these scaffolds. Results showed that the releasing of bFGF from the scaffolds significantly promoted cell proliferation and helped capillary formation. Moreover, combing two or more growth factors and controlling their spatio-temporal release could be another option for improving the functionality of scaffolds.
Many studies have also reported the immobilization of VEGF-mimetic peptides on scaffolds as a potential solution for vascular regeneration. D’Andrea et al. designed a VEGF-mimetic peptide, QK (domain: KLTWQELYQLKYKGI), and showed the ability to activate VEGF receptors and similar bioactivity to VEGF [25]. QK peptides provided many advantages, including low molecular weight, low immunogenic potential, and cost-effectiveness by synthesis [25][26][27][28][29]. Leslie-Barbick et al. reported that QK peptides were easier to conjugate or immobilize into scaffolds than VEGF because they could diffuse into scaffolds faster and more completely [29]. Another study loaded QK peptide into poly(ethylene glycol)-b-poly(L-lactide-co-e-caprolactone) (PELCL) nanofibers by emulsion or suspension electrospinning [30]. It was found that QK loaded PELCL electrospun scaffolds could significantly accelerate the proliferation of ECs compared with pure PELCL scaffolds in nine days. Zhou et al. functionalized the surface of a PELCL scaffold with QK peptides via EDC/NHS chemistry [28]. In vitro studies demonstrated that the QK peptide-functionalized PELCL scaffolds could significantly promote the proliferation of ECs compared with unfunctionalized PELCL scaffolds. QK peptide-functionalized electrospun scaffolds showed their ability of fast endothelialization, which could have potential use in vascular regeneration.
Apart from VEGF-memetic peptides, RGD peptide was also reported to immobilize into electrospun scaffolds for vascular tissue engineering. For example, Kim et al electrospun a mixture of PLGA and PLGA-b-PEG-NH2 to generate electrospun scaffolds with a functionalizable amine [31]. RGD was covalently grafted with on PLGA fibrous scaffolds. In vitro study showed that the immobilization of RGD significantly promoted cell adhesion and proliferation. These results suggest that RGD functionalized fibrous scaffolds could be promising for vascular regeneration.
Hydrogen sulfide (H2S), a unique gasotransmitter that has been recognized as an important physiological and pathological signaling molecule, can mediate and promote the effects on angiogenesis [32][33]. The phenomenon that H2S promotes EC proliferation and migration has been reported by different groups [34][35]. H2S has been reported to simulate angiogenesis in vitro and in vivo [34][36]. Since H2S has been recognized to be beneficial for angiogenesis, researchers started to focus on the development of H2S releasing scaffolds for vascular regeneration [37][38]. Feng et al. electrospun N-(benzoylthio)benzamide (NSHD1), an H2S donor, with PCL solution to form H2S release fibrous scaffolds [39]. The H2S fibrous scaffolds could facilitate (H9c2 and 3T3) cell proliferation. Moreover, these scaffolds were also reported to increase the expression of collagen type I and collagen type III, and wound healing related genes. Kang and his colleagues synthesized a pH-controlled H2S donor (JK-1) from phenylphosphonothioic dichloride [40]. Wu et al., instead, mixed JK-1 with PCL to prepare H2S releasing electrospun nanofibers [41]. The fabricated fibrous scaffolds showed that lower pH induced greater and faster H2S release. JK1 doped PCL fibers were nontoxic to fibroblasts and in vivo experiments proved that PCL-JK1 could significantly improve wound healing. Although previous studies showed promising results in vitro and in vivo, many aspects still need to be improved for the fabrication of H2S releasing nanofibrous scaffolds: (1) a slow H2S-releasing donor needs to be included; (2) in addition to physical doping, other surface functionalization approaches should be considered.
The chick chorioallantoic membrane (CAM) assay and rabbit or rat corneas are the most widely used animal models for studying the process of angiogenesis in vivo. CAM is an extraembryonic membrane mediating gas and nutrient exchanges until hatching [42]. Since this membrane could form blood vessels network after incubation, it has been widely performed as an in vivo model to screen angiogenesis stimulators and inhibitors in response to biomaterials. Rabbit cornea has been reported to be another in vivo angiogenesis model. However, CAM is believed to be simpler and more cost-effective with lower ethical concerns than other animal models.
CAM assay has been used by many researchers as an in vivo model in vascular tissue engineering for more than 40 years. A number of studies have evaluated electrospun nanofibrous scaffolds on the CAM to examine their angiogenic response and biocompatibility. The general procedure of CAM assay is shown in Figure 1. By implanting the scaffolds onto the CAM, their potential angiogenic activities can be analyzed through changes in the vascular density of the surrounding environment. Test components can be growth factors, peptides, drugs, and other biomolecules, which are immobilized or incorporated on nanofibrous scaffolds. For example, in order to evaluate the angiogenic ability of VEGF-loaded collagen-PCL scaffolds in vivo, a CAM assay was carried out, showing that VEGF-loaded PCL scaffolds could significantly increase the vessel area on the scaffolds [43] Their result proved that the VEGF released from VEGF-loaded scaffolds could promote early blood vessel formation in vivo. Augustine et al. developed zinc oxide (ZnO) nanoparticle-loaded electrospun PCL scaffolds [44]. A 1 wt% ZnO nanoparticle incorporated PCL scaffold was pro-angiogenic, and it proved to have statistically more branching points than PCL scaffolds in a CAM assay. Diaz-Gomez et al. performed a CAM assay to evaluate the angiogenesis response of platelet-rich plasma (PRP)-coated PCL scaffolds [45]. PRP-PCL scaffolds were integrated with the CAM and formed many capillary blood vessels around the PRP-PCL scaffolds compared to the PCL scaffolds. These few selected studies are just to exemplify the potential of the CAM assay as a simple validation tool for newly designed scaffolds for vascular tissue regeneration, after an initial assessment in vitro. For a more thorough review on the several angiogenesis available assays, the reader is referred elsewhere [46].
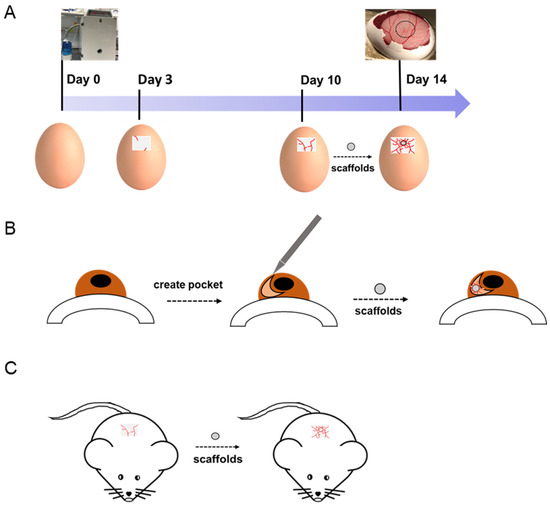
Figure 1. (A) Chick chorioallantoic membrane (CAM), (B) rabbit cornea, and (C) mouse models are used to evaluate the angiogenic abilities of implanted scaffolds.