Exosomes as nanosized vesicles are emerging as drug delivery systems for therapeutics owing to their natural origin, their ability to mediate intercellular communication, and their potential to encapsulate various biological molecules such as proteins and nucleic acids within the lipid bilayer membrane or in the lumen. Exosomes contain endogenous components (proteins, lipids, RNA) that could be used to deliver cargoes to target cells, offering an opportunity to diagnose and treat various diseases. Owing to their ability to travel safely in extracellular fluid and to transport cargoes to target cells with high efficacy, exosomes offer enhanced delivery of cargoes in vivo. However, several challenges related to the stabilization of the exosomes, the production of sufficient amounts of exosomes with safety and efficacy, the efficient loading of drugs into exosomes, the clearance of exosomes from circulation, and the transition from the bench scale to clinical production may limit their development and clinical use. For the clinical use of exosomes, it is important to understand the molecular mechanisms behind the transport and function of exosome vesicles.
1. Introduction
Over decades, synthetic drug delivery systems such as liposomes, micelles, dendrimers, and polymeric nanoparticles have been exploited to improve the efficacy and therapeutic index (in terms of the pharmacokinetics and pharmacodynamics profiles) of therapeutics, while minimizing the toxicity and drug-related off-target side effects
[1][2][3][4]. Many hurdles still exist for synthetic drug delivery systems, including the delivery of drugs to target organs, toxicity owing to the chemical and physical features of the synthetic delivery system, reactions to the host immune system, and activation of an acute hypersensitivity reaction, which can result in discontinuation of treatment in some individuals. The utilization of extracellular vesicles as a natural carrier system to deliver therapeutics can overcome the limitations associated with synthetic drug delivery systems
[1][5][6][7].
Extracellular vesicles are differentiated into apoptotic bodies, microvesicles, and exosomes, depending on the intracellular origin and size. Apoptotic bodies contain cellular contents, including deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and histone proteins, with sizes ranging from 50 to 5000 nm and which are formed by membrane blebbing during apoptosis. Microvesicles, also known as microparticles or ectosomes, have sizes ranging from 50 nm to 1000 nm and are formed through fission from plasma membranes. Exosomes are nanosized membrane vesicles with a size range of 30–100 nm, which are secreted by various types of cells
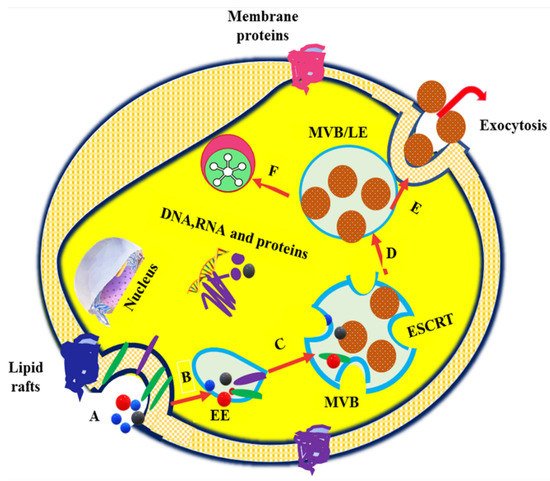
[8][9][10][11]. Exosome formation typically involves the formation of endocytic vesicles from the plasma membrane; inward budding of the endosomal vesicle membrane, which results in the multivesicular body (MVB); and the release of exosomes derived from the MVB into the extracellular environment when the MVB fuses with the plasma membrane
[12]. The exosome biogenesis, cargo sorting, and vesicle release processes are shown in . Owing to their nanoscale dimensions, exosomes have received greater attention in recent years and are considered to be the most promising vehicles for drug delivery to target cells or organ
[13].
Figure 1. Exosome vesicle formation, cargo sorting (starts from endocytosis and ends in the MVBs), and release. (
A). Endocytosis of the plasma membrane (
B) Uptake of proteins, nucleic acids, and membrane-associated molecules into encysted body and formation of EE. (
C) Transformation of early endosomes (EE) into multivesicular bodies (MVB) or late endosomes (LE), intraluminal vesicle (ILV) formation via inward budding of MVB or LE, and cargo sorting through ESCRT- and ESCRT-independent pathways. (
D) Fusion of MVB or LE with the plasma membrane and lysosome. (
E) Exocytosis of exosomes in response to MVB–plasma membrane interactions. (
F). Degradation of MVB or LE by the lysosome (modified from
[14]).
Exosomes are released by cells in both physiological and pathological circumstances. As exosomes circulate in the blood, they may operate as signal transducers both locally and far away from their source
[15]. For example, exosomes are released via activation at sites of vascular damage, where they may have a signaling or adhesion function. Antigen-presenting cells also secrete exosomes that carry peptide-loaded MHC molecules functioning as intercellular vehicles for antigenic materials
[16][17]. Several studies have reported that exosomes have been found to be released from several regions of the female reproductive system, including the endometrium, uterus, oviduct epithelium, placenta trophoblastic cells, and preimplantation embryos. Exosomes have critical roles in modulating transcription and translational activity, granulosa cell proliferation and differentiation, cumulus expansion, gametogenesis, proper follicular growth, oocyte maturation, fertilization rate regulation, embryo development, and blastocyst formation and implantation, as well as pregnancy outcomes and fertility
[18][19][20][21][22][23]. The presence of exosomes in reproductive system secretions further indicates their potential functions in preconception and postconception intercellular interactions; nonetheless, modified exosomes have recently been employed as markers for pregnancy and diseases linked with pregnancy in humans
[23][24][25].
Exosomes are involved in sperm activities and epigenetic inheritance and are secreted by the epididymis (epididymosomes) and prostate (prostasomes). There are two types of epididymosomes: CD9+ epididymosomes and ELSPBP1-enriched epididymosomes. The CD9+ epididymosomes govern sperm maturation by transferring their protein cargoes to the spermatozoa. ELSPBP1-enriched epididymosomes bind to dead spermatozoa preferentially, quenching reactive oxygen species that could otherwise harm sperm maturation
[26]. Protein cargoes in prostasomes, as with those in epididymosomes, are transported to spermatozoa and are involved in sperm survival and motility via calcium-dependent signaling. Despite the fact that the prostasome contains DNA, coding, and regulatory RNAs with potential modulatory activities, there is little indication that these nucleic acids are transferred to spermatozoa
[27]. Choy et al. isolated and characterized the testicular exosomes, demonstrating that they were taken up by somatic and germ cells, including sperm cells. Their findings have provided new insights into intercellular communication in the testes, which have broad implications for spermatogenesis and paternal epigenetic inheritance
[28]. Proteins in the exosomes may be associated with cell recognition, allowing them to target a certain cell type. The majority of epididymosome-associated proteins are transferred to the subcellular or membranous sperm domains during epididymal transit and are involved in the acquisition of fertilization ability, modulation of motility, and protection against oxidative stress. Proteins associated with prostasomes stimulate sperm motility and regulate the capacitation timing to prevent premature acrosome response induction
[29]. In a previous study, Choy et al. revealed the plethora of proteins in the testicular exosomes that have been implicated in male fertility, suggesting that the communication mediated by testicular exosomes is required for spermatogenesis
[28].
Exosomes play an essential role in intercellular communication by carrying genetic and proteomic information between neighboring cells or distant organs. Exosomes communicate their message or deliver the cargo to the recipient cell in different ways. Firstly, exosomes bind to target cell membranes through ligands expressed on their surfaces, facilitating the ligand–receptor interaction. This ligand–receptor interaction elicits an immune response and mediates hemostasis, angiogenesis, and cancer progression
[30]. Secondly, exosomes transfer their surface proteins and cytoplasms to the target cells through budding and subsequent fusion with the plasma membranes of the target cells
[31][32]. The third mechanism involves the horizontal transfer of proteins and genetic material from one cell to another. Studies have shown that fusion or internalization of exosomes can aid in the transfer and release of their cargo and in mediating regulatory processes
[8][33]. Despite the wide range of functions carried out by exosomes, little is known about the molecular pathways involved in exosome secretion. Exosome release is aided by the presence of Ca
2+. In most cell types, an increase in the intracellular Ca
2+concentration, a universal intracellular signal, is required to initiate exosome secretion. During the exocytosis process, the membrane of a secretory vesicle fuses with the plasma membrane in a tightly controlled Ca
2+-triggered reaction. In endocrine cells, secretory granules contain many Ca
2+ ions, and it has been suggested that a high intragranular Ca
2+ concentration is required for effective exocytosis
[17]. Therefore, it is evident that many intracellular transport events depend on Ca
2+, and thus it is likely that Ca
2+ might be required for the fusion events involved in the secretion of the exosomes
[34].
Exosomes have many properties that a drug delivery vehicle should have, such as tolerability due to their wide distribution in biological fluids, the ability to exert functional responses by transferring their cargoes across the membranes of the target cells, the potential to mediate intercellular transfer of mRNAs and miRNAs, and the ability to cross biological barriers
[35][36][37]. Various cell types are used to obtain exosomes, including mesenchymal cells, immune cells, and tumor cells. The selection of the cell type is crucial because the exosomes’ functions (quantity of drug load, amount of exosome release) will depend on the properties of the cell types. Moreover, the biodistribution of exosomes may vary depending on the cell origin
[38].
In exosome formulation, biomolecules including coding and non-coding RNAs and cell-targeting and cell adhesion moieties are packed within the lumen or lipid bilayer. The exosome structure is similar to the unilamellar liposome, whereby an amphiphilic lipid bilayer surrounds an aqueous core. Due to this structural resemblance, there are expected to be similar characteristics between exosome and liposome drug delivery systems
[11]. However, the low immunogenicity, non-cytotoxicity, and non-mutagenicity of exosomes give them superior drug delivery potential compared to liposomes
[39]. For these reasons, exosomes can be used as specific drug delivery systems by selecting the exosome source. Exosomes possess different roles, acting as innate bio-therapeutics, therapeutic targets, and drug delivery carriers. Several approaches can be used to maximize the efficacy of exosomes, including introducing exogenous drug molecules, increasing their innate therapeutic capability, and altering their surfaces to improve their in vivo bio-distribution and attenuate their pathological activities
[40].
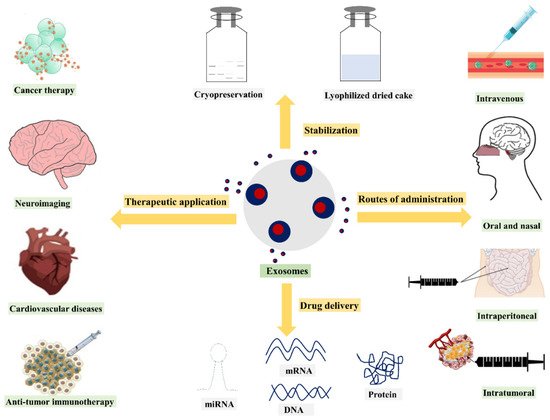
To date, researchers have mainly utilized exosomes as drug or gene carriers, disease markers, and therapeutic targets. In recent years, various methods have been used for exosome isolation and characterization. Numerous studies have demonstrated that depending on the different cell types, exosomes containing different compositions exhibit different functions. As a result, the current review discusses exosome drug delivery systems from the following perspectives: exosome isolation or purification and characterization methods; exosome applications as drug, gene, and nucleic acid delivery systems; the various administration routes of exosomes, as well as their production methods and scalability challenges. Additionally, clinical trials and regulatory challenges related to exosome delivery systems will be reviewed and discussed. An overview of the use of exosomes for drug delivery, their clinical applications, and their potential administration routes with stabilization strategies is presented in .
Figure 2. Overview of drug delivery and therapeutic applications of exosomes.
2. Exosomes in Drug Delivery
Typically, liposomes and polymeric nanoparticles are the most preferred drug delivery systems for entrapment or encapsulation of drug molecules, and these delivery systems are routinely used to deliver different classes of drug molecules, including anticancer, antifungal, and analgesics molecules. However, the biocompatibility, better stability, long-term safety, and ability to evade the host immune system with long systemic circulating capability and stability remain major concerns
[6][12].
Over the past few years, considerable efforts have been made to develop exosomes as novel nanoscale delivery systems. Several characteristics of exosomes, including their biocompatiblity and biodegradability, mean they do not elicit acute immune reactions, have low toxicity, carry the required fusogenic properties, contain low-uptake machinery, have high specificity to the target cells, and are smaller in size, making exosomes attractive nanocarrier drug delivery systems. Further, exosomes have a tendency to accumulate more in tumor tissues than in normal tissue. Moreover, the specificity of the exosome delivery platform can be further enhanced by anchoring exosomes with tumor targeting ligands such as proteins, peptides, or antibodies for specifically targeted drug delivery
[41][42]. shows the application of exosomes for therapeutic delivery in various pathological conditions.
2.1. Protein and Peptide Delivery:
Exosomes represent a favorable therapeutic approach for delivering protein or peptide molecules. Initially, exosomes were investigated as a garbage bin to remove the proteins, lipids, and nucleic acids that are unwanted for cells. Over the past few years, exosomes have been known to convey biological molecules for various diagnostic and therapeutic purposes. Exosomes isolated from most of the cells are intrinsic carriers for endogenous protein molecules, suggesting that the exosome carrier system would be a suitable delivery approach for proteins or peptides
[43]. Proteins such as enzymes, transmembrane proteins, and cytoskeletal proteins are reported to be delivered by exosomes. The exosome vesicles have been considered as a transporter of biomolecules, including lipids, proteins, and genetic material, owing to their ability to transfer their contents from mother cells to neighboring cells
[8][44].
Naturally occurring exosomes contain membrane-associated protein ligands. Upon their biogenesis, these bioactive ligands cluster into microdomains on the exosomes, thereby providing a natural membrane environment for the biomacromolecules, which helps maintain stability and bioactivity, thereby contributing to elevating the efficiency of membrane protein therapeutics
[45][46].
A number of proteins, such as heat shock proteins, annexins, and Rab family proteins, which are abundantly found in exosomes, are mainly involved in trafficking of exosomes and intercellular assembly, and inclusion of such proteins in exosomes may not be beneficial for drug delivery purpose. However, several other protein therapeutics may be exploited for exosome delivery purposes
[35].
In the treatment of cancer, Survivin-T34A (dominant-negative mutant of the inhibitor of apoptosis protein Survivin) was successfully introduced into exosomes isolated from melanoma cell lines and played an important role in inducing apoptosis
[47]. Kooijmans et al. anchored antiepidermal growth factor receptor nanobodies to the surfaces of exosome vesicles via glycosylphosphatidylinositol to improve the interactions between exosomes and epidermal growth factor receptor-expressing tumor cells
[48].
Modified exosomes derived from the dendritic cells consisting of SAV (a protein that binds to biotin with high affinity) and LA (an exosome-tropic protein) were mixed with pH-sensitive GALA peptides to produce GALA-modified exosomes. These engineered exosomes are effective in controlling the intercellular transport and antigen presentation ability of tumor cells
[49]. Similarly, alphagalactosylceramide or ovalbumin-loaded exosomes could potentially induce an adaptive immune response in the absence of triggering invariant natural killer T-cell anergy
[50].
Tian et al.
[51] conjugated exosomes on the surface of the c(RGDyK) peptide using bio-orthogonal chemistry for the treatment of ischemic stroke by targeting the lesion region of the ischemic brain. Further, these engineered exosomes were loaded with curcumin to suppress both the inflammatory response and cellular apoptosis in the targeted (lesion) region. The in vivo results for the cRGD–exosome delivery system showed encouraging therapeutic efficacy and targeting ability.
Some biomolecules are prevalent in all types of exosomes, including the generation of cytosolic proteins such as tubulin and actin, protein kinases, Annexin and Rab family proteins, tetraspanins (CD9, CD63, CD81), heat shock proteins (HSP 70, HSP 90), and various transmembrane proteins molecules
[52]. Exosomes derived from antigen-presenting cells carry tetraspanin CD86 and major histocompatibility complex molecules I and II on their surfaces, enabling them to simulate CD8
+ and CD4
+ T cells. In addition, some molecules present at the exosome membrane may act as pathogen-associated molecular patterns, thereby contributing to the activation of immune cells
[53]. Exosomes have the ability to increase and modulate immune responses, which could be one important strategy in the design of new vaccine formulations. The nanoscale exosomal vesicles are capable of stimulating innate and adaptive arms of the immune system, suggesting their potential to activate granulocytes or NK cells, and are also able to interact with CD8
+, CD4
+, and B cells in order to demonstrate antigen-specific immune responses
[54].
In a previous study, Sandra et al.
[55] investigated exosomes as potential vaccine adjuvants. Exosomes were isolated from lipopolysaccharide endotoxin (LPS)-stimulated human monocytic cell line (THP-1). The isolated exosomes were combined with a hepatitis B recombinant antigen (HBsAg) solution or suspension comprising HBsAg-loaded poly-ε-caprolactone–chitosan nanoparticles. The obtained results suggested that exosomes combined with HBsAg induced a humoral immune response similar to the control group, which is a HBsAg solution without exosomes. The findings of their study suggest that exosomes, when co-ingested with the antigen, could have potential applications to improve the protective immune response in vaccine development.
Liu et al.
[56] investigated the utility of exosomes in neuronal recovery after ischemic stroke. Enkephalin-loaded exosomes containing a transferrin complex called enkephalin-tar-exo were developed to target the blood–brain barrier (BBB). In vivo delivery of the exosomal system showed that enkephalin-tar-exo crossed the BBB and decreased the levels of lactate dehydrogenase, p53, and caspase-3 when tested in rats using a transient middle cerebral artery occlusion–reperfusion model. In addition, the enkephalin-tar-exo system improved the brain neuron density and neurological score, indicating neurological recovery after stroke.
Barok et al.
[57] examined the delivery of an antibody–drug conjugate (trastuzumab–emtansine) against HER2-positive cancer. Exosomes were isolated from several cell lines such as HER2+ (SKBR-3 and EFM-192A breast cancer), HER2 (MCF-7 breast cancer), and gastric cancer (SNU-216) via ultracentrifugation method followed by treatment with trastuzumab–emtansine. The results showed that antibody–drug-conjugated exosomes bound to HER2+ cancer cells with growth inhibition and activation of caspases-3, confirming the binding of trastuzumab–emtansine to HER2+ cancer cells.
Cho et al.
[45] compared the efficacy between exosomes and the ferritin nanocage carrier in the delivery of signal regulatory protein α owing to the greater phagocytosis of tumor cells by macrophages, whereby the tumor growth inhibition induced by the exosome carrier was higher than that of nanocages. The abundance of proteins and lipids in the exosome vesicles offers a substantial advantage in providing an ideal microenvironment for membrane proteins regarding the activity and distribution in the membrane, reflecting their potential advantage over other delivery platforms.
Although exosome-associated proteins play an important role in triggering cellular responses and regulatory processes, the functional aspects of exosomes are complex in terms of their assembly, binding, fusion with targeted cells, and interactions with the extracellular matrix; for instance, paraformaldehyde-mediated crosslinking of proteins on the surfaces of exosomes decreased fusion of exosomes with parental cells by approximately 20%. Furthermore, exosomes that were treated through solubilization with octylglucoside and reconstructed by dialysis to remove the membrane proteins showed a decrease in their ability to fuse with target cells compared to untreated exosomes
[58]. The fusion efficiency of exosomes with depleted proteins demonstrated comparable fusion efficiency to that of large unilamellar vesicles whose lipid composition was similar to that of naturally occurring exosomes, confirming the importance of exosome-associated proteins in fusion events
[8]. Kim et al.
[59] evaluated the potential of genetically modified exosomes to express a targeting ligand that can improve the exosome delivery to a target tissue and reduce the systemic toxicity. Briefly, the ability of cardiac-targeting peptide, a targeting ligand expressed from genetic modification of exosomes, to deliver tissues and heart cells in vitro and in vivo was investigated. Exosomes isolated from HEK293 cells via differential centrifugation were genetically modified by fusion of cardiac-targeting peptide (CTP)–Lamp2b on the membrane of the exosome (CTP–Exo), and exosomes expressing only Lamp2b (CTL–Exo) were used as a control. The in vitro study results showed that compared to CTL–Exo, a significant increase in the delivery of CTP–Exo was observed in H9C2 rat cardiomyocytes. In vivo studies showed that delivery of CTP–Exo was 15% more effective than CTL–Exo, confirming that genetic modification of exosomes with targeting peptides can be explored as a therapeutic tool for heart diseases owing to enhanced delivery and reduced systemic toxicity. Recently, for successful delivery across the BBB, exosomes loaded with the antioxidant protein catalase showed an improved disease state in Parkinson’s disease patients. As with many other actives, delivery of catalase across the BBB is a major hurdle, however loading of catalase into the exosome carrier system seems to be a promising approach for Parkinson’s disease therapy.
In a previous study, Haney et al.
[60] incorporated catalase into exosomes using different methods, including incubation with or without the use of saponin permeabilization, freeze–thaw cycles, and extrusion and sonication procedures. Western blot analysis showed that sonication and extrusion techniques are the most effective in incorporating the catalase into exosomes. Further, lipophilic fluorescent dye was used to label the exosomes to confirm the delivery of catalase across the BBB. The exosomes labeled with fluorescent dye were incubated with a pheochromocytoma (PC12) cells. Confocal images confirmed the uptake of labeled exosomes in PC12 cells. When exosomal catalase was added, elimination of reactive oxygen species (ROS) was observed in in vitro-activated macrophages, suggesting successful delivery of exosome-loaded catalase and neutralization of ROS.
2.2. Exosomes in Gene Delivery
The triggering of RNA interference to induce gene silencing has been significantly intensified in biomedical applications. The RNA interference technique involves processing double-stranded RNAs into small interfering RNAs (siRNAs) via posttranscriptional, sequence-specific gene silencing. The siRNAs are used to selectively cleave target mRNA
[61][62][63]. Several RNA-structure-related factors, including their negative charge, instability in the blood circulation owing to degradation by nucleases, immunogenicity, and need for a delivery vehicle, particularly when repeated dosing is needed to treat disease, limit the biomedical application of synthetic siRNAs
[64][65]. The use of exosomes that can carry exogenous siRNAs to human cells can overcome these impediments. Exosomes as naturally occurring RNA carriers might be an effective source for the delivery of genetic material
[66]. Owing to their natural ability to carry genetic material such as DNA and RNA to the targeted cells, exosomes have attracted increased interest in drug delivery involving genetic modification or alterations of gene expression in certain genetic therapies. Typically, small interfering RNAs (siRNAs) are used to disrupt the gene of interest. However, these siRNAs are prone to rapid degradation in the systemic circulation owing to their low stability. Exosomes as a carrier system help in both the protection and delivery of siRNAs to the targeted cells. Several studies have reported the utility of exosomes as therapeutic vehicles for the delivery of exogenous genetic material. There is a shortage of safe, efficient, target-specific therapeutic delivery vehicles for conveying genetic material. In the past, the ability of exosomes to transport endogenous mRNAs and microRNAs expressed by the exosome-producing cells to different cells in culture has demonstrated the concept of using exosomes for gene delivery
[12].
Exosomes were used to deliver siRNA due to being non-immunogenic to the host and owing to their natural ability to deliver RNA from cell to cell. Wahlgreen et al.
[67] used human exosomes to deliver siRNA to T cells and monocytes. Exosomes were isolated from various cell types, including lung cancer cells TB-177 and HeLa cells, then siRNA was loaded into exosomes via chemical transfection and electroporation methods. The results from confocal microscopy, flow cytometry, and Western and Northern blotting showed successful incorporation of siRNA into exosomes. Additionally, whether the delivered exosomes induced posttranscriptional gene-silencing in recipient cells was evaluated by performing immunoblotting analysis. The results showed that exosome-loaded siRNA caused a decrease in tagged siRNA against mitogen-activated protein kinase 1 (MAPK-1) expression, indicating successful gene slicing with downregulation of the specific gene, with these results suggesting the utility of exosomes as delivery vehicles in gene therapy. Similarly, Shtam et al.
[68] evaluated the potential of exosomes to deliver siRNA to human cells by targeting RAD51, a gene protein that helps in repairing double-strand breaks of DNA. Exosomes isolated from HeLa cells using the centrifugation method were loaded with Alexa Fluor 488-labeled siRNA and then further co-cultured with HeLa and HT1080 cells. The results from the Western blot analysis showed that exosome-delivered siRNA downregulated the expression of RAD51 and RAD52. Skog et al.
[69] reported that exosomes obtained from glioblastoma tumor cells could be used for diagnosis as they naturally contain miRNA in the exosomes. Using exosomes, the delivery success rate of siRNA was enhanced significantly. Moreover, exosomes released from the self-derived dendritic cells of mice had a more than 60% success rate in delivering siRNA specifically to the nervous system, which is much greater than that of the siRNA itself
[70].
Exosomes isolated from different types of cells may have different compositions and functions. Exosomes released from endothelial cells are mostly associated with atherosclerosis and vascular inflammation. However, the ability of exosomes to deliver exogenous contents has not been explored greatly. In a study, Banizs et al.
[71] investigated the potential of endothelial exosomes to deliver siRNA to endothelial cells. Exosomes isolated from endothelial cells using filtration and ultracentrifugation methods and then further loaded with siRNA using the electroporation technique were investigated for their capability to deliver siRNA to endothelial cells. Further, exosomes carrying siRNA were incubated in the presence of luciferase-expressing endothelial cells. The study results showed that endothelial-exosome-loaded siRNA expressed lower luciferase compared to the control group, suggesting the functionality of endothelial exosomes to deliver exogenous material to cells in vitro at the target site.
Typically, nucleic acid drugs can be loaded either endogenously or externally. The ability of nucleic acid drugs to exert a maximum therapeutic effect is important for vigorous exploration, gene expression, and maintenance of the physiological balance of the cells regulated by the delivery of specific functional siRNA to target cells. However, it is difficult for exogenous siRNA to penetrate the cell membrane and it can be easily degraded. One major challenge for the clinical application of gene therapy is the development of a suitable vehicle for diffuse delivery of genetic material to the brain. The ability of exosomes to load exogenous genetic cargo, the specificity imparted by the targeted exosomes, and their potential to systematically administer genetic material and invoke immune evasion are important properties for gene therapy applications
[70][72][73].
Lydia et al.
[70] loaded exogenous siRNA using dendritic-cell-derived exosomes through electroporation method, while engineering of dendritic cells was performed to express Lamp2b, an exosomal membrane protein, in order to achieve tissue-specific targeting. Further, the exosomes were injected with the targeting peptide RVG into mice intravenously to deliver GAPDH siRNA to neurons, oligodendrocytes, and microglia in the brain to show a specific gene knockdown. The study results showed that the exosome vesicles delivered GAPDH siRNA specifically to neurons in knockdown-related genes. The loading of exogenous linear DNA by exosomes using electroporation technique to explore the potential of delivering DNA to recipient cells in the clinical gene therapy was previously reported. However, the capacity of DNA and its loading efficiency depended on both the size of the DNA and the exosomes
[74]. The delivery of nucleic acid drugs or genetic material via the exosomes involves fundamental treatment at the genetic level, which has gained greater attention in treating several diseases. Nevertheless, the usage methods, precise mechanism, and clinical effects in relation to safety considerations still need to be vigorously explored
[49].
In recent years, studies have reported the successful delivery of siRNA to target cells. Faruqu et al.
[75] loaded siRNA into exosomes for delivery to cancer cells. Exosomes isolated from HEK-293 cells by centrifugation method were fluorescently labeled and then loaded onto exosomes by electroporation method. The excess siRNA after loading into exosomes was removed using gel filtration. The results showed the efficient encapsulation of siRNA with promising exosome yield and successfully delivery into cancer cells.
Similarly, Limoni et al.
[76] developed LAMP2B-DARPin-bearing exosomes to specifically bind to HER2/Neu, with subsequent delivery of the siRNA molecule against the TPD52 gene into SKBR3 cells. The results showed that exosome-loaded siRNA downregulated the gene expression of TPD52 by up to 70%, indicating the successful gene transfer to cancer cells by exosomes, providing an additional delivery system option for gene therapy. Zhang et al.
[77] evaluated the efficiency of serum-derived exosomes in delivering siRNA via intratracheal instillation into lung macrophages to modulate lipopolysaccharide-induced lung inflammation. The results indicated that the exosome delivery system avoided the immune system, which is a major concern in delivering genetic material.
2.3. Exosomes in Delivery of Other Therapeutic Compounds
The vast majority of studies have investigated the exosome-based drug delivery systems for therapeutic transfer of interfering RNAs and other therapeutic cargo, while exosomes isolated from cancer cells for anticancer drug delivery systems have been explored for the potential loading of anticancer agents into exosomes. Exosomes are also promising delivery vehicles for small-molecule drugs owing to their small size, reduced toxicity, and bio-compatibility compared to nanoformulations such as liposomes and dendrimers. Drugs (particularly anticancer drugs) encapsulated in exosomes have demonstrated improved pharmacokinetic and pharmacodynamic properties and enhanced in vivo anticancer activity compared to free drugs
[78]. Several studies have explored the use of exosomes in the in vitro and in vivo delivery of small-molecule drugs. Few studies have reported the superior therapeutic effects of exosome-loaded small-molecule therapeutics compared to free drugs or encapsulated carriers
[79]. Schindler et al.
[80] developed doxorubicin-loaded exosomes and studied their redistribution and rapid cellular uptake to the cytoplasm and nucleus. The doxorubicin exosomes demonstrated enhanced in vitro potency in multiple cell lines with increased cell uptake and redistribution when compared to free doxorubicin and its liposomal formulations. The exosome delivery system can provide benefits for both cell-based and nanotechnology-based drug delivery, allowing efficient transport of drugs, which can overcome the various biological barriers. Nevertheless, several factors need to be considered before using the exosomes for transporting drug molecules, such as the efficient drug loading of exosomes without causing significant changes in the exosomal membrane structure or content.
In cancer therapy, exosomes have the capability to interact with and accumulate in the target cancer cells. The exosomes found in Taxol are taken-up via endocytosis, owing to the presence of adhesion proteins, immunoglobulins, proteoglycans, tetraspanins, integrins, and lectins, meaning exosomes have superior uptake
[81]. Furthermore, cellular membranes found in exosomes may fuse with endocytic membranes to deliver drugs, overcoming the Pgp-mediated efflux. Additionally, exosome-mediated cell-to-cell communication is important in the encounters between cancer cells and the immune system
[82]. Parolini et al.
[58] reported that the fusion of exosomes with target cells occurred more efficiently under acidic conditions, indicating the preferential uptake of exosomes by tumor cells, which have an acidic microenvironment, as compared to the surrounding healthy tissue. Since the exosome vesicles are small in size and native to the animals, they can be used to avoid phagocytosis and bypass the engulfment by lysosomes. Thus, most chemotherapeutic agents such as doxorubicin and paclitaxel are encapsulated into exosomes, showing potential for delivering into target cells
[12].
Kim et al.
[83] evaluated the feasibility of exosomes in delivering paclitaxel for multi-drug-resistant cancer. Various methods, including incubation at RT, electroporation, and mild sonication, were used to incorporate paclitaxel into exosomes. Of these methods, mild sonication provided the greatest loading capacity, which could be because a decrease in rigidity and the microviscosity of exosomal membranes upon sonication allowed the incorporation of paclitaxel into lipid bilayers of exosomes. The study results showed that paclitaxel-loaded exosomes greatly increased the cytotoxicity of drug-resistant MDCKMDR1 (Pgp+) cells, indicating the therapeutic potential of exosomes to treat drug-resistant cancers. The mechanisms likely to be responsible for the enhanced exosome-loaded anticancer activity included the efficient transport of paclitaxel into the target cancer cells, overcoming the Pgp-mediated drug efflux in the cells resistant to cancer, and the preferential accumulation of paclitaxel in targeted cancer cells.
In the past, curcumin encapsulated in exosomes has been shown to be more stable, being highly concentrated in blood and with a therapeutic effect
[84]. Curcumin exhibits antioxidative and anti-inflammatory properties and could be a promising treatment for cerebral diseases. Accumulating evidence suggests that curcumin, when encapsulated in exosomes, induces exosome secretion and increases its solubility, stability, and therapeutic potential
[85][86].
Kalani et al.
[87] investigated the potential of exosomes when primed with curcumin in terms of endothelial cell dysfunction by studying their effects on oxidative stress, tight junction proteins, and endothelial cell layer permeability. The results demonstrated that oxidative stress in Hcy-treated cells was significantly decreased with exosome-loaded curcumin, suggesting its antioxidative potential. Likewise, curcumin-primed exosomes showed a beneficiary effect in amelioration of junction proteins and endothelial cell layer permeability by maintaining redox homeostasis, lowering the levels of MMP-9 and improving tight junctions, which eventually improved the endothelial cell permeability. In another study, exosomes were incorporated with curcumin to enhance the effectiveness of curcumin. Exosomes derived from a murine tumor cell line (EL-4) were mixed with curcumin and then subjected to sucrose gradient centrifugation. TSG101 and CD81 were used as exosomal protein markers to identify the exosome curcumin complex. Various in vitro and in vivo experiments were carried out to assess the anti-inflammatory activity of exosomal curcumin. The macrophages treated with exosome-loaded curcumin showed fewer inflammatory cytokines in vitro than those treated with curcumin alone, suggesting enhanced anti-inflammatory activity caused by the exosomal curcumin. In vivo, mice treated with exosomal curcumin demonstrated significant survival in a lipopolysaccharides-induced septic shock animal model compared to mice treated with curcumin alone
[84].
Although exosomes have been proven to be useful carriers for delivering anticancer drugs, their in vivo delivery of anticancer drugs could be limited owing to their non-specific toxicity and off-target effects, which can be observed with the conventional delivery of chemo drugs. This necessitates the anchoring of tumor-targeted ligands such as peptides, antibodies, and aptamers with drugs loaded onto exosome surfaces to reduce the non-specific toxicity and allow tumor-specific drug delivery. Such ligand-equipped exosomes were reported to exhibit tumor growth suppression via a receptor-mediated endocytosis process, thereby overcoming endosome encapsulation and trapping
[88].
Liu t al.
[89] tested surface-modified exosomes with ligands to deliver doxorubicin. Exosomes isolated from the non-cancerous HEK293T cells were anchored with a ligand (lipophilic hyaluronic acid) to target CD44-overexpressing MCF7/ADR breast cancer cells. The ligand-anchored exosomes were shown to be elevated by doxorubicin in breast cancer cells, thereby decreasing the tumor mass by 89%. In addition to improving the properties of small-molecule drugs, exosomes are also used to transport drugs across the BBB. Owing to the problems associated with the permeability of small-molecule drugs across the BBB, most of the potent central nervous system drugs have not been successful in clinical trials because the majority of these drugs cannot cross the BBB. To compensate for these complications, exosomes as a body’s own cells are employed as delivery vehicles to tailor the drugs to cross the BBB, enhancing drug transport to the brain by decreasing mononuclear phagocyte system drug clearance
[12][90].
Table 1. Examples of exosomes as drug delivery systems.
| Cargo Type |
Origin of Exosomes |
Disease Type |
Isolation or Purification Method |
Drug Loading Method |
Outcome |
Reference |
| Proteins |
Signal regulatory protein α |
Human embryonic kidney293T cells |
Cancer |
Centrifugation |
Transfection |
Enhanced phagocytosis of tumor cells |
[45] |
| Survivin-T34A |
Melanoma cell lines |
Pancreatic cancer |
Centrifugation |
NA |
Apoptotic death of cells |
[91] |
| Antiepidermal growth factor receptor |
Mouse neuroblastoma |
Epidermoid carcinoma |
Ultrafiltration/size exclusion liquid chromatography |
NA |
Target specificity |
[48] |
| 20S proteasome |
Mesenchymal stem cells |
Mouse myocardium |
Tangential flow filtration |
NA |
Reduction in myocardial infraction |
[92] |
| Genetic substances |
miRNA |
Glioblastoma cells |
Glioblastoma tumor |
Differential centrifugation |
Transfection |
Providing diagnostic information |
[69] |
| |
miRNA |
Human cord blood endothelial colony-forming cells |
Ischemic kidney injury |
Centrifugation |
Transfection |
Protected kidney function and reduced kidney injury |
[93] |
| |
Spherical nucleic acids |
PC-3 cells |
Prostate cancer |
Centrifugation |
Naturally
encased |
3000-fold-enhanced knockdown of miR-21 |
[94] |
| |
siRNA |
Human embryonic kidneycells (HEK293) |
Breast cancer |
Sequential centrifugation |
Electroporation |
TPD52 gene expression was downregulated up to 70% compared with non-targeted exosomes |
[76] |
| Small molecules |
Paclitaxel |
Prostate cancer cell lines (PC-3 and LNCaP) |
Autologous prostate cancer |
Differential centrifugation |
Co-incubation |
Enhanced drug cytotoxicity to cancer cells |
[95] |
| Doxorubicin |
Immature mouse dendritic cells transfected with the vector-expressing iRGD-Lamp2b fusion proteins |
Breast cancer |
Centrifugation and ultrafiltration |
Electroporation |
Specific drug delivery to the tumor site andinhibited tumor growth |
[79] |
| Curcumin |
Tumor cells (GL26-Luc, BV2, 3T3L1, 4T1, CT26, A20, and EL-4) |
Brain tumor and autoimmune encephalitis |
Sucrose gradient centrifugation |
Direct mixing |
Inhibited brain inflammation and delayed brain tumor growth |
[84] |
| Dopamine |
Kunming mouse blood |
Parkinson’s disease |
Ultracentrifugation |
Co-incubation |
Enhanced therapeutic effect due to brain specific drug delivery |
[96] |
3. Exosomes Drug Loading Techniques
The lipid bilayer membrane of the exosome vesicle serves as a natural barrier to protect the degradation of cargo in the blood circulation. However, this lipid bilayer membrane, as well as the endogenous content of exosomes, makes drug loading into exosomes challenging
[97]. Generally, active loading and passive methods can be used to sort the drug into the exosomes
[98]. Active loading is also known as remote or postdrug loading, in which the drug is incubated with isolated exosomes. Passive drug loading, also termed as the preloading method, involves the secretion of drug-sorted exosomes from a pretreated donor or source cells. This method does not require the addition of drugs into the exosome vesicle. The active loading approach has been reported to be more effective in attaining a higher drug/vesicle ratio owing to its active pumping mechanisms. The postloading approach is more suitable for hydrophobic drugs than hydrophilic drugs
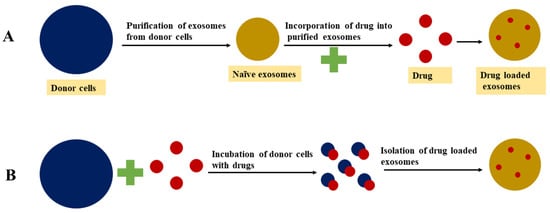
[99][100]. Different approaches for drug loading into exosomes are presented in , while the advantages and disadvantages of different exosome drug loading approaches are detailed in .
Figure 3. Exosomal drug loading approaches: (A) postloading approach; (B) preloading approach.
3.1. Passive Loading Approach
3.1.1. Incubation of Drugs with Exosomes
This approach is also known as the passive drug loading method, in which both drug and exosomes are incubated together and the drug diffuses into exosomes along the concentration gradient. The drug loading efficiency using this method is directly related to the hydrophobicity of drug molecules because of the potential of hydrophobic drugs to interact with the lipid bilayer membrane of the vesicle
[100]. In a study, Dongmel et al. incubated mouse lymphoma-derived exosomes with curcumin in PBS at 22 °C for 5 min, then the mixture was centrifuged based on a different sucrose gradient. The encapsulation of curcumin into exosomes enhanced the solubility, stability, and bioavailability compared to free curcumin
[84]. Similarly, Vashisht et al.
[101] reported that incubation of curcumin with exosomes resulted in a loading efficiency of 70.46%. Enzyme catalase was also encapsulated into exosomes via incubation in PBS at room temperature for 18 h. However, the low loading capacity is one of the main drawbacks associated with this method
[60].
3.1.2. Incubation of Drugs with Donor Cells
In this approach, the targeted exosome donor cells are treated with a drug molecule of interest and the pretreated cells then secrete drug-loaded exosomes
[14]. The objective of this approach is for the donor cells to accumulate the bioactive or therapeutic compounds and secrete exosomes that can accommodate the therapeutic compounds. Owing to its untargeted nature, this approach may result in low exosome yield
[100]. Pascucci et al.
[102] treated and incubated SR4987 mesenchymal stromal cells with a low dose of paclitaxel for 24 h. Then, the cells were washed and reseeded in a new flask containing a fresh medium. After 48 h of culture, the exosomes loaded with paclitaxel were isolated and collected from the cell-conditioned medium. The pretreated donor cells may be exposed to mechanical or biological stimuli such as ultraviolet light, heat, or combination in order to release the drug-loaded exosomes
[103][104].
3.2. Active Drug Loading Approaches
Active drug loading involves temporary disruption of the exosome membrane so that active cargo can easily diffuse into the vesicles. The integrity of the exosome membrane is then restored after the desired compounds are loaded into the exosomes. The various approaches used to disrupt the membranes of the exosomes include sonication, extrusion, and freeze–thaw cycles
[98]. Compared to passive drug loading, the drug loading capacity of exosome vesicles increased up to 11 times using the active drug loading approach
[99]. The main problem associated with this approach is the potential to damage targeting features and the native structure of exosomes during the membrane disruption process
[98].
3.2.1. Sonication
Exosomes derived from donor or target cells are mixed with a drug or protein of interest and then sonicated using a homogenizer probe. The mechanical shear force generated during sonication disturbs the exosome membrane’s integrity and allows bioactive compounds to diffuse into the exosome while deforming the membrane
[100][105][106]. Kim et al.
[83] reported that the microviscosity of the exosome membrane decreases significantly after sonication. However, the membrane-bound proteins or lipid contents of the exosome are not significantly affected by this membrane deformation process. It was found that the membrane integrity of the exosome can be restored within an hour when incubated at 37 °C. Moreover, in some cases, biphasic drug release is observed from the exosomes when the drugs are encapsulated inside of the exosomes and also attached to the outer membrane layer of the exosome vesicle. When the drug is attached to the exosome outer layer this results in burst release, whereas when the drug is encapsulated inside the exosomes this leads to slow release
[83].
3.2.2. Extrusion
Extrusion is a postloading method that employs a syringe-based lipid extruder for drug loading. Exosomes isolated from the donor cells are mixed with a targeted drug and then loaded into a syringe-based lipid extruder with a 100–400 nm porous membrane at a controlled temperature. During the extrusion, the drug is vigorously mixed with the disrupted exosome membrane
[14]. Fuhrmann et al.
[99] reported the benefits of the extrusion approach for the drug loading of exosomes. Exosomes derived from MDA-MB231 breast cancer cells were loaded with porphyrin using the extrusion method. Compared to the incubation method, extrusion loading resulted in a greater cytotoxic effect. Further, the extrusion method alters the zeta potential of original exosomes, while increasing the number of extrusions in the intensive extrusion process can contribute to effective drug loading due to the transformation of the vesicle constitution.
3.2.3. Freeze–Thaw Cycles
Drug loading using the freeze–thaw approach involves incubation of exosomes with a targeted drug at room temperature for a given amount of time and rapid freezing at −80 °C or in liquid nitrogen. Then, the mixture is thawed at room temperature. For better drug encapsulation, freeze–thaw cycles are repeated for at least three cycles. This method has lower drug loading capacity compared to sonication or extrusion approaches. Additionally, this technique can promote the aggregation of exosomes, leading to broad size distribution of the drug-loaded exosomes
[60][100][107].
3.2.4. Electroporation
Electroporation utilizes an electric field, which facilitates the movement of drug molecules into the lumen of the exosomes by disturbing the phospholipid bilayer of the exosomes, thereby creating pores on it
[108]. During electroporation, drug molecules diffuse through the pores formed on the exosome lipid bilayer membrane; meanwhile, the integrity of the membrane is recovered after the loading. This method is widely used for the loading of large molecules such as nucleotides (siRNA or miRNA) into exosomes
[99]. The electroporation technique has low loading capacity owing to RNA aggregation and exosome instability issues. This technique can increase RNA loading into exosomes and enhance the loading of hydrophilic small molecules into exosomes
[99].
3.2.5. Incubation with Membrane Permeabilizers
Membrane permeabilizers and surfactants such as saponin can interact with the cholesterol in the cell membrane and form pores, which leads to exosomal membrane permeability. Compared to the incubation method, the membrane permeability method can enhance the loading efficiency of catalase into exosomes
[109]. A previous study showed that an 11-fold increase in drug loading of hydrophilic molecules into exosomes was observed with saponin compared to the passive loading approach without saponin
[99][100]. Using this method, the amount of saponin used for drug loading should be optimal and exosomes should be purified after incubation with saponin.
| Drug Loading Approach |
Mechanism |
Advantages |
Disadvantages |
| Passive loading |
Incubation of exosomes and free drugs. |
Diffusion of cargo into a cell or exosomal membrane. |
Simple operation.
Does not compromise the membrane integrity. |
Loading efficiency.
Drugs may cause cytotoxicity to the donor cells. |
| Incubation of the donor cells with free drugs. |
| Active loading |
Sonication |
Creation of micropores for diffusion by mechanical shear force. |
Higher loading capacity than the simple incubation method. |
Sonication-induced membrane damage is a roadblock for large scale application. Influence on exosome integrity and cargo aggregation. |
| Extrusion |
Membrane recombination. |
High cargo loading efficiency.
Repeated extrusion provides a homogeneous blend of exosomes with cargoes. |
Recombination of exosomal surface structure may compromise the immune-privileged status of exosomes, making exosomes visible to immune cells such as mononuclear phagocytes. |
| Freeze–thaw cycles |
Membrane fusion. |
Simple and effective strategy to load various cargoes (drugs, proteins, and peptides) into exosomes directly. |
Repeated freeze–thaw may cause protein degeneration and exosome aggregation.
Drug loading efficiency is lower than sonication and extrusion methods. |
| Electroporation |
Creation of micropores for diffusion by the electric field. |
High loading efficiency |
The loading efficiency and aggregation of cargoes are major limitations. |
| Incubation with membrane permeabilizers |
Dissolves membrane molecules (cholesterol), create pores on the exosomal surface. |
Higher loading capacity as compared with the simple incubation method |
Saponin is hemolytically active in vivo, limiting the concentration (toxicity) of saponin used for drug loading.
Extra purification process may be required to remove saponin. |