1000/1000
Hot
Most Recent

Polyphenols are natural organic compounds produced by plants, acting as antioxidants by reacting with ROS. These compounds are widely consumed in daily diet and many studies report several benefits to human health thanks to their bioavailability in humans. However, the digestion process of phenolic compounds is still not completely clear. Moreover, bioavailability is dependent on the metabolic phase of these compounds. The LogP value can be managed as a simplified measure of the lipophilicity of a substance ingested within the human body, which affects resultant absorption. The biopharmaceutical classification system (BCS), a method used to classify drugs intended for gastrointestinal absorption, correlates the solubility and permeability of the drug with both the rate and extent of oral absorption. BCS may be helpful to measure the bioactive constituents of foods, such as polyphenols, in order to understand their nutraceutical potential. There are many literature studies that focus on permeability, absorption, and bioavailability of polyphenols and their resultant metabolic byproducts, but there is still confusion about their respective LogP values and BCS classification.
Phenolic compounds (PCs) are secondary plant metabolites, characterized by an aromatic ring and several attached hydroxyl groups. These compounds offer protection to the plant from pathogens, free oxygen radicals, UV rays, and parasites [1].
Polyphenols represent a large and varied group of at least 10,000 known different compounds that could be unified by the presence of one or more aromatic rings with one or more hydroxyl groups in their chemical structure [2][3]. For some plant products, for example, some exotic fruits or cereals, the composition of polyphenols is still poorly known [4]. Regarding dietary PC, the current known compounds are about 8000 variants and they are naturally found in common fruits, vegetables, and beverages and, according to the number of phenolic rings they contain, they could be classified into main four subclasses: [5] flavonoids, including flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols; phenolic acids subclass, which is divided between those compounds derived from hydroxybenzoic acids, such as gallic acid, and those derived from hydroxycinnamic acid, like caffeic, ferulic, and coumaric acid; and stilbenes and lignans (Figure 1) [6][7]. In addition to these, there are other subclasses that are not included among the currently known, i.e., alkylphenols, curcuminoids, furanocumarins, hydroxybenzaldehydes, hydroxybenzoketones, tyrosols, and so on [8].
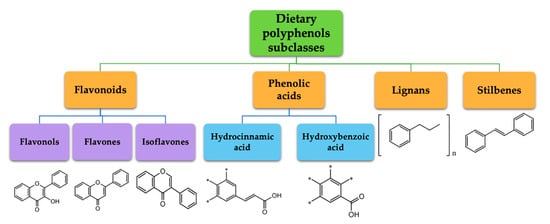
Figure 1. Dietary polyphenols known subclasses scheme.
PCs are derived from a common biosynthetic pathway, involving precursors from the shikimate and/or the acetate–malonate pathways [9]. In addition, their role in the prevention and improvement of human health has been widely demonstrated. There is growing evidence to support antioxidant, anti-inflammatory, anti-tumoral, and anti-cardiovascular disease roles attributable to polyphenols [10][11][12][13][14][15][16][17][18]. There are numerous different types of PCs, all characterized by different chemical structures that have distinct properties [19]. It was reported that the specificity of the health benefits, conferred by a single PC, is based on specific chemical classes [20].
As PCs are bioactive compounds, it is important to study their distribution within the human body. More specifically, in order to express their therapeutic effect, PCs should undergo pharmacological metabolism reactions, with consequent conversion into more soluble metabolites, and subsequent excretion [21][22]. From this perspective, it is important to study the health potential of PCs, and this may be done through the biopharmaceutical classification system (BCS). This classification evaluates the capacity of drugs (and thus also bioactive compounds) to pass through lipidic biological membranes as well as to interact with aqueous solutions during their metabolism within the human body. Hence, BCS allows to characterize the health capacities of a PC.
The aim of this work is to analyze the nutraceutical potential of 10 dietary PCs, in terms of intestinal absorption, permeability, solubility, and BCS classification of these compounds, and to compare them with the literature currently available, in order to establish an association between all these factors. Furthermore, it was chosen to focus this work only on 10 dietary PCs because they belong to most of the subfamilies of known polyphenols, and thus can be representative for an overall evaluation of the beneficial prospective of dietary PC present in nature. Besides, in the current literature, there is enough and exhaustive information on permeability, solubility, and especially BCS classification only on these PCs, univocally directing the discussion of this review to them.
In mammals, PCs are subject to both phases I and II of drug pharmacokinetic metabolism, respectively, as represented in Figure 2. PCs are ingested mainly in a conjugated form, as O-glycosides (step 1, Figure 2). Metabolism of glycosylated PC is initiated in the oral cavity [22], after contact with the glycosidase enzymes of oral microflora, as demonstrated by Kamonpatana et al. [23] for anthocyanidins. However, most PCs continue intact along the digestive tract [22]. On arrival in the stomach and inside the small intestine mucosa, the glycosides are converted by a hydroxylation reaction into their corresponding aglycones (phase I drug metabolism) (step 2, Figure 2). This reaction is assisted by β-glucosidase enzymes expressed by the intestinal microbiota. In this way, aglycones may pass from the gut lumen to the cytosol of the enterocytes, predominantly by passive diffusion (step 3, Figure 2), or by protein carriers, such as P-glycoprotein (P-gp) and co-transporters for sodium-glucose transporter (SGLT1) [24][25][26]. Some hydroxycinnamic acids, such as ellagitannins, are resistant to enzymatic digestion in the small intestine and, therefore, pass directly to the colon, where they are metabolized by microbiota into aglycones [27]. Once the aglycones have been absorbed into the enterocytes or colon cells, they move through the portal vein (step 4, Figure 2) to the liver, where they are further conjugated (phase II drug metabolism) to become O-glucuronides and O-sulphates (step 5, Figure 2). A variable portion of the phenolic conjugates is then excreted into the bile and re-enters the small intestine to undergo the metabolic cycle once again [28][29][30]. Finally, the resultant phenolic conjugates (O-glucoronides/O-sulphates) are transported to the bloodstream by plasma proteins until they are excreted in urine [31][32][33].
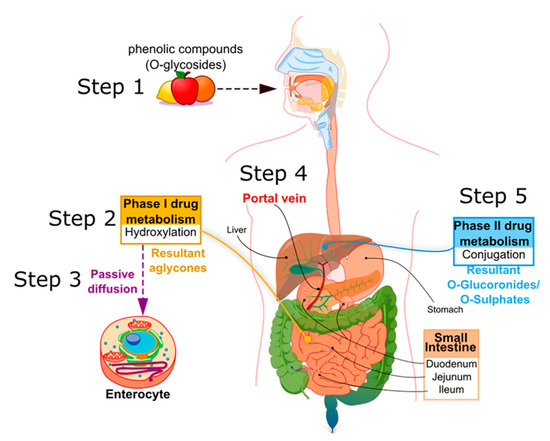
Figure 2. Principle metabolic steps of dietary phenolic compound (PC) in humans.
Biotransformation, during the metabolic processes in humans, is determined by the structural characteristics of the specific phenolic compound [9]. This is because the chemical structure of the compound is specific in promoting the action of only selected intestinal enzymes and gut microbiota species. It has been shown that gut microbiota are involved in the release of phenolic aglycones and hepatic O-glucuronides [28][34]. According to the different structural subfamilies, PCs undergo intestinal bio-transformations by specific microbiota families [31][35][36][37][38][39][40]. Furthermore, it was observed that each single phenolic compound metabolized generates numerous metabolite byproducts, usually two or three, but also many more, i.e., glycosylated quercetin produces up to 20 metabolites. According to Del Rio [41], all these modifications during absorption have a profound influence on the biological activity of the resultant phenolic metabolites, as these may play an active role within different pathways in the human body. An example is the activation of the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) [42]. It was shown that protocatechuic acid, a metabolite from anthocyanins, is a known Nrf2 activator [43][44]. Likewise, caffeic acid metabolites have Nrf2 activating properties [45][46][47]. In addition, another protective function pathway was found to be influenced by phenolic metabolites; it was shown that methylated scutellare presents an inhibitory effect on H2O2-induced cytotoxicity in PC12 cells, thus indicating protective activity [48]. In order to be able to perform all the metabolic reactions, it is necessary that PC, as well as all xenobiotics, administered orally, enter the intestinal epithelium to reach the blood and lymphatic circulation [49]. The transcellular mechanisms required to permit the entry of these compounds into the intestinal mucosa are as follows: passive diffusion, carrier-mediated active facilitated transport, and paracellular transit in tight junctions [50][51]. Nevertheless, generally, the majority of drugs enter the cells by passive diffusion [52]. PCs, characterized by low molecular weights, and that are sufficiently hydrophobic and non-charged, are permitted to be transported by passive diffusion [24]. This involves the production of biliary salts and the formation of micelles, which permeate through the translocation of the apical membrane of the enterocytes [53]. Some PCs, such as hydroxytyrosol, tyrosol, p-cumaric acid, apigenin, and luteolin, are selectively combined in micelles and absorbed differently [54]. In addition, in an in vitro study using Caco-2 cells to test the permeability of six dietary polyphenols (caffeic acid, chrysine, gallic acid, quercetin, resveratrol, and rutin), it was shown that several chemical-physical features are related to the passive diffusion transport capability of molecules through cells. These factors include the lipophilicity (expressed as partition coefficient logarithm, LogP), molecular weight, ionization state the number of rotatable bonds (RB), and number of hydrogen-bonding acceptor/donor (HBA/HBD), respectively [55]. Moreover, it was observed that the scarcely lipophilic ferulic acid (FA) passes through transcellular transport, by tight junction [56]. Regarding structurally complex PCs, such as gallotannins and ellagitannins, hydrolysis processes permit conversion into smaller molecules, thereby facilitating assimilation in simpler forms by enterocytes. However, this conversion reaction cannot occur in the small intestine, so they pass directly into the large intestine where they are fermented by the microbiota and then can be absorbed by passive diffusion at the level of the colon [9][57][58]. All these transport mechanisms influence the bioavailability of these compounds in humans [49].
Bioavailability is a term used in pharmacokinetic language to indicate the fraction of the drug that reaches the systemic circulation without any chemical modification. Recently, it has also been used for food nutrients. According to the current accepted definition, bioavailability is the proportion of the nutrient that is digested, absorbed, and metabolized through normal pathways [54].
Each class of PC has its own unique chemical structure that results in specific solubility and lipophilicity, which in turn affects the bioavailability. These parameters influence both the rate and degree of absorption of phenolic metabolites in the human body during metabolism, before being incorporated into plasma circulation [59]. In the studies of Marrugat [60], Fito [61], and Tian [62], the bioavailability of various PC was analyzed by detecting plasma and urine concentrations after the ingestion of pure compounds or food with a known phenolic content. It was found that phenolic concentrations in either plasma or urine were not directly related to the respective concentrations in target tissues, but were instead dependent on their metabolic form. This is explained by the fact that PC, although ingested in a glycosylated form, undergo metabolic processes, such as hydrolysis/hydroxylation by the intestinal enzymes and subsequent conjugation with either glucuronic or sulfonic acid in the liver. This leads to a change in their chemical structure, thereby modifying the respective lipophilicity and solubility characteristics, permitting entry into the blood circulation. Therefore, PCs are found in the blood circulation in conjugated forms. To confirm this, quercetin and daidzein aglycones were not found in either plasma or urine after they are ingested, but in their conjugated form (after phase II metabolism) [63]. Resveratrol represents an exception, as it undergoes glycosylation in order to protect the compound from oxidative degradation. Hence, the glycosylate resveratrol is chemically more stable and soluble, and consequently more easily absorbed in the human gastrointestinal tract [64]. Finally, it was shown that large size conjugated metabolites are eliminated in bile, while small conjugates, such as monosulphates, are preferably excreted in urine [59].
In this perspective, BCS classification may allow prediction of health effects for PC contained in foods and thus are administered mostly orally. In 2000, the Food and Drug Administration (FDA) purposed the BCS system as an approach to avoid in vivo tests when drugs are also characterized by rapid dissolution [65]. More in particular, it was highlighted that high permeability of a drug does not limit the absorption of a compound during its transit in the intestinal system. In addition, high solubility of a drug will not limit its dissolution and consequently neither its absorption, thus the gastric emptying process is the only limiting step for the absorption of a highly soluble and highly permeable compound [66]. However, some studies have shown that the FDA’s BCS guidance, despite supporting studies on bioavailability through in vitro test such as on Caco-2 [66], may not always be enough to correctly predict the extent of drug absorption in humans [67][68].
To study the bioavailability of an active compound, the most reliable measure is the area under the plasma drug concentration curve versus time (AUC). AUC is directly proportional to the total amount of unchanged drug that reaches systemic circulation. Plasma drug concentration increases with the extent of absorption, and the maximum plasma concentration is reached when the drug elimination rate equals the absorption rate. The time peak, which is reached when maximum plasma drug concentration occurs, is the most widely used general index of absorption rate; the slower the absorption, the later the peak time. To determine significant AUC values, a cutoff of 80% was defined [69]. For dietary supplements, herbs, and other nutrients, such as PC, in which the administration is nearly always oral, bioavailability can be identified as the quantity or fraction of the ingested dose that is absorbed [70]. Hence, in this review, the maximum plasma concentration and time peak of the PC examined will be taken into consideration for the evaluation of their bioavailability.
In the early study of Lempereur [71], it was seen that the source of hydroxicinnamic acids in foods is relatively varied. For instance, FA is the most abundant phenolic acid found in cereal grains and represents up to 90% of the total polyphenol content. In the study of Zhao [72], it was shown that FA has a very high bioavailability in rats [73][74]. It was observed that FA, after undergoing absorption by intestinal epithelial cells and conjugation reactions [75], was present in both plasma and urine mainly in its conjugated form [76]. In several clinical studies carried out on rats, FA administrated at 8 μmol kg−1 [72], and between 6 and 15 mg kg−1 [77][78][79], displays high plasma concentration between 8174.55 ng L−1 and 0.444 mg L−1. Higher results were detected with a higher dosage (20 mg kg−1) [80], with a peak plasma concentration of around 12 mg L−1, whereas it seems that even higher doses (0.5 and 1.5 g kg−1) lead to a maximum plasma concentration of around 12 ng mL−1, showing a limiting rate of absorption. Furthermore, FA time peak was observed at less than 1 h, which implied that it is rapidly absorbed in rat plasma after oral administration [81].
Caffeic acid is the most representative hydroxycinnamic acid present in nature, and can be found in food (mostly fruits) as well as in its ester form, as chlorogenic acid [19]. Clinical studies observed that chlorogenic acid is rapidly absorbed and metabolized by the intestine, thus the digestion metabolites may be detected in plasma between the first 5 min and 1 h after ingestion in rats [82][83]. The current literature showed that chlorogenic acid absorption is dependent on administered doses, i.e., a low administered dose (1–100 mg kg−1) [83][84][85] leads to a concentration between 0.55 and 91 ng mL−1 of caffeic acid in plasma concentration; likewise, higher doses (400 mg kg−1 from Lonicerae Japonicae Flos extracts) [82], for which peak plasma concentration was registered at around 1500 mg mL−1, show an absorption rate dependent on the ingested dose. Regarding urinary excretion, the concentration of chlorogenic acid found after 24 h from ingestion was around 30–34%, indicating that this compound is rapidly eliminated as well as it is rapidly absorbed [83].
The most studied flavonols are rutin and quercetin, and they are mostly found in buckwheat, asparagus, and citrus fruits, but also in peaches, apples, and green tea [86][87]. In pharmacokinetics studies carried out on rats and human volunteers [88][89][90][91][92], low bioavailability of rutin was shown, due to its hydrophilic nature, thus suggesting that it cannot diffuse easily through cell plasma membranes. To be absorbable, rutin needs to undergo to hydroxylation into quercetin. In fact, after oral administration of rutin (328 μmol kg−1), only quercetin sulfates and glucuronides were detected in serum, with a concentration of 2 and 5 nmol mL−1 [89]. This evidence was in line with previously observations, where, after oral rutin administration (500 mg), the absorption rate was 40–200 ng mL−1 of quercetin [88] and showing the metabolism changes that occur on this compound, which are necessary to absorb it within the human body.
Quercetin bioavailability was found to be very poor, as it is rapidly metabolized in the human body; therefore, in the conjugated form (quercetin metabolite), its beneficial capacities are limited compared with the aglycon form [91]. Furthermore, the total quercetin conjugates measured in plasma concentrations after oral ingestion are very low. In the study of Dong [90], rats showed rapid absorption of quercetin (8.51 mg kg−1) from Matricaria chamomilla L. extract, with a final plasma concentration around 0.29 µg mL−1 detected after 0.79 h (47 min) from ingestion, confirming that it is rapidly absorbed after oral administration. In Graefe study [91], human volunteers who ingested 100 mg of quercetin glycosides showed a maximum plasma concentration at 2.12 µg mL−1 and urine concentration at 4.5%. This evidence confirmed the rapid absorption of quercetin aglycone, which is supposed to occur in the upper part of the intestine, thus involving active absorption mechanisms. Kaşıkcı [93] demonstrated that absolute bioavailability (2.01 μM) of quercetin was attained after ingesting the compound suspended in aqueous solution.
Regarding flavones, celery, red peppers, chamomile, mint, parsley, rosemary, oregano, traditional Chinese herbs, and ginkgo biloba are the major sources of this subclass [94]. It was shown that apigenin administered at both low doses (13.82 mg kg−1) [90] and high doses (60 mg kg−1 and 100 mg kg−1) [95][96], is similarly absorbed, with max plasma concentration registered between 0.14 and 1.33 μg mL−1. Moreover, in the study carried out on six men, the apigenin conjugates detected in urine after 24 h from the administration lead to an excretion rate around 0.22%, suggesting that most of the apigenin ingested is rapidly metabolized or is excreted unabsorbed, thus showing high permeability of this PC within the human body [97].
Following the flavones subclass, cirsimaritin, mostly found in rosemary and oregano, was also investigated. In a pharmacokinetic study on rats [98], 8 mg kg−1 of cirsimarin (glycosyde form) was administered from crude extract of Microtea debilis and, 5 h after ingestion, the low permeability of this PC was demonstrated: the determination of plasma concentrations of cirsimaritin aglycone was 0.138 µM and the urine concentrations after 5 h from oral administration were only 5.05 µM (3–5%). These results showed that cirsimarin is not absorbed from the gastrointestinal tract, but in the stomach, and then it must be converted to cirsimaritin to produce systemic healthy effects within the human body.
Isoflavones are present almost exclusively in leguminous plants, particularly daidzein, which is found in large quantities in soybeans and soymilk [99]. Based on the several studies carried out on rats or human volunteers, daidzein showed low bioavailability. In fact, in experiments with both low concentration (from 0.4 to 1 mg kg−1) [100][101] and high concentration of oral administered daidzein (from 30 to 50 mg kg−1 and 418 μmol L−1) [102][103][104], the serum peak registered (173.1 ng mL−1 and from 0.38 to 2.5 μmol L−1) appeared within 2 or 8 h from ingestion [102], highlighting its rapid absorption from the gastrointestinal tract, and indicating the low bioavailability of this compound.
Despite the low quantities of stilbenes in the human diet, resveratrol is both the most representative polyphenol and the widely studied, as it is considered the main one responsible for health benefits [105][106][107]. More in particular, it was indicated by numerous recent studies that resveratrol presents several benefits to human health, such as antibacterial, antioxidant, anti-inflammatory, and anticancer activities [108][109][110][111]. Resveratrol has been detected in numerous plants, particularly in red grapes, and thus is highly concentrated in red wine and grape juice [112]. Regarding bioavailability of this compound, after oral administration, resveratrol is absorbed by passive diffusion or by membrane transporters within the intestine, where it undergoes metabolic reactions, and then the resulting metabolite is released in the bloodstream, where it can be detected [113]. It was demonstrated from several clinical studies that, after oral dose of resveratrol between 25 and 150 mg [114][115][116][117][118][119], the max plasma concentration registered ranges between 491 ng mL−1 and 471 μg L−1. Moreover, lower doses of resveratrol were studied (0.5 and 1 mg) [120] and a low plasma concentration of the glucuronidated form at 130.19 ng mL−1 was registered, whereas for higher doses (from 500 mg to 5 g) [119][120][121][122], the plasma concentration detected was between 0.5 μg mL−1 and 4 μg mL−1. These several studies demonstrated that the absorption rate is dependent on the orally administered dose of resveratrol. Urinary excretion of this PC and its metabolites was rapid, with 77% of all urinary agent-derived species excreted within 4 h after the lowest dose [121]. As previously explained, when resveratrol is taken orally, it is metabolized to its glycosylated form, which increases its stability and solubility, allowing this compound to be more easily absorbed [64]. Hence, it was concluded that the systemic bioavailability of resveratrol is high, showing high permeability within the human body, thus accumulation of potentially active resveratrol metabolites may produce healthy effects within the human body [115].
Ellagic acid is a natural phenolic antioxidant found in many fruits and vegetables, such as walnuts, pecans, cranberries, raspberries, strawberries, grapes, peaches, and pomegranates. Clinical studies carried out on this compound showed its low bioavailability. More in particular, both low oral administration (20–25 mg) [123][124] and high oral administration (85.3 mg kg−1 and >500 mg) [125][126] showed a plasma concentration in human patients between 30 and 200 ng mL−1. An exception can be made for the 40 mg dose, which can be considered halfway between a low and high administered dose. The serum peak concentration was registered around 200 ng mL−1, which was similar to the serum peak concentration obtained from a high dose of ellagic acid (>500 mg). These results showed that the absorption system of ellagic acid becomes saturated above a certain dosage of this PC and, therefore, the maximum detectable plasmatic concentration seems to reach its plateau, which appears to be around 200 ng mL−1 in the case of ellagic acid. Moreover, this peak concentration in the serum was reached after 1 h from the administration, owing to the fact that 50% of total ellagic acid was shown to bind to blood proteins after intestinal absorption (within the first 30 min after oral administration) [127].
Among Curcuminoids, curcumin covers relevance in Southeast Asia, as it is abundantly found in turmeric, which is a spice widely used in Southeastern Asian countries’ culinary traditions [128]. It is relevant to note the highly lipophilic nature of curcumin (high LogP), attributable to the methine-rich segments that connect the polar regions [129]; this is also reflected in its capacity to interact with biomembranes [130][131]. However, its therapeutic potential is still debated owing to its poor bioavailability in humans. Curcumin has been shown to have low permeability and to be poorly absorbed from the small intestine, whereas conjugative metabolism in the liver is extensive [129]. The low availability is also caused by the binding of curcumin to enterocyte proteins, altering its chemical structure [132]. It was seen that curcumin is ineffectively transported through the intestinal mucosa into circulation [133]; this molecule can undergo biotransformation within the intestinal mucosa or directly in the bloodstream [134][135].
Pharmacokinetic studies demonstrated that a high dosage of this compound, between 8 and 12 g daily [128][136][137][138][139], displayed a low plasma concentration, ranging between 50 ng mL−1 [139][140] and 2 μg mL−1 [128], and similar with low dosages (from 100 mg to 4 g daily) [136][137][140][141][142][143][144][145][146], with peak plasma concentrations registered around 0.51 nM [136][143], 15.8 nmol L−1 [140], and 12.2 and 96 ng mL−1 [144][145][146]. In both studies of Mahale [143] and Dhillon [139], the maximum plasma concentration was detected 2 h after ingestion of curcumin and urinary levels collected after 24 h were 210 and 510 nmol L−1 of curcumin glucuronides [140], showing that its metabolites have a short time period within the human body, meaning curcumin is not expected to be able to exert its beneficial health effects.