In order to investigate whether acetazolamide enhances the therapeutic efficacy of cisplatin and/or cisplatin/fendiline, we assessed the combined activity of these drugs on tumor growth in vivo, treating NOD-SCID mice which had been subcutaneously xenografted with SKNBE2 NB cells, and evaluated tumor growth rate and animal survival.
The mice were divided into different experimental groups treated with: DMSO 1% in NaCl saline solution 0.9%, as vehicle control; cisplatin (5 mg/kg, Cis); acetazolamide (20 mg/Kg, AZ); fendiline hydrochloride (3 mg/kg, Fen); cisplatin in co-administration with acetazolamide (Cis/AZ); cisplatin in co-administration with fendiline hydrochloride (3 mg/kg, Cis/Fen); cisplatin in co-administration with fendiline hydrochloride and acetazolamide (Cis/Fen/Az).
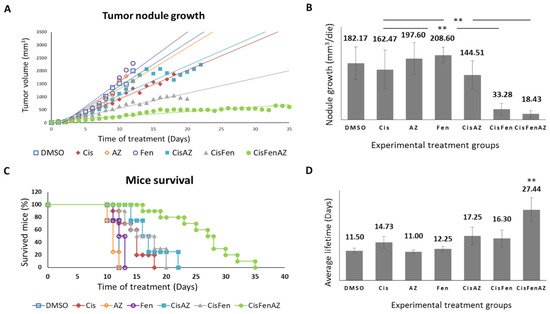
Figure 1 reports the growth rate of tumor nodules and mice survival in the different experimental groups. Single treatments with acetazolamide or fendiline per se do not reduce tumor nodule growth, as shown by the daily recordings of nodule volume, which did not differ from DMSO-treated controls; cisplatin alone, at the dose used, had a moderate effect, but, in line with our previous results
[7], the combined cisplatin/fendiline treatment reduced tumor growth much more effectively than low-dose cisplatin-based chemotherapy; conversely, no enhancing effects were observed in the co-treatment with cisplatin and acetazolamide, which induced an antitumor effect comparable to cisplatin alone. Interestingly, in the mice treated with the combination therapy which included all three molecules (Cis/Fen/Az), there was a reduced nodule growth rate equal to approximately one tenth of that observed in the vehicle-treated control mice, and if compared with the Cis/Fen treatment, the growth rate halved (
Figure 1A,B). Moreover, it was observed that the disease was stable in these mice for up to 35 days, suggesting a sustained activity of the triple-drug combination. Other groups were sacrificed upon reaching the established 2.2 mm
3 mass volume threshold of the nodule.
Figure 1. Tumor nodule growth and mice survival. Once SKNBE2 mass reached 5 mm in diameter, mice were treated with DMSO, Cisplatin 5 mg/kg (Cis); acetazolamide 20 mg/Kg (AZ); fendiline hydrochloride 3 mg/kg (Fen); cisplatin 5 mg/kg in co-administration with acetazolamide 20 mg/Kg (CisAZ); cisplatin 5 mg/kg in co-administration with fendiline hydrochloride 3 mg/kg (CisFen); cisplatin 5 mg/kg in co-administration with fendiline hydrochloride 3 mg/kg and acetazolamide 20 mg/Kg (CisFenAZ). The graphs depict linear trend lines of nodule tumor volume (A) and average growth of the nodule per day (B) in each experimental group. In (C), the Kaplan–Meier plot indicates the percentages of mice survival at increasing days after the beginning of treatments. A highly statistically significant decrease of mass growth is induced by CisFen and CisFenAZ treatments (** p < 0.001). The histograms in (D) represent the average lifetime of mice for each treatment group. A highly statistically significant increase of survival is induced by CisFenAZ (** p < 0.001). To compare differences between experimental groups, statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey post hoc test.
In addition, the survival of Cis/Fen/Az-treated mice was prolonged compared to all the other experimental groups. Kaplan–Meier survival curves were analyzed using the Logrank test. Cis/Fen/Az-treated mice showed a highly significant difference in survival when compared to all other groups, on average they survived 27.44 days (p < 0.001), whereas Cis/Fen- and Cis/Az-treated mice registered an average survival of 16.3 and 17.25 days respectively, thus only slightly longer and with no statistical significance when compared to treatment with cisplatin alone (14.7 days) (Figure 1C,D). Altogether, these results demonstrate that acetazolamide might ameliorate the chemotherapeutic approach for HR-NB, enhancing the antiblastic effects of low-dose cisplatin and constituting a possible novel anticancer agent to be administered in combination with cisplatin and fendiline hydrochloride.
In addition, in order to clarify whether the action of acetazolamide was restricted to the type of neuroblastoma cell line we used, we verified the same additive effect in vitro on two different lines (IMR32 and SH-SY5Y) and obtained the same effects (see Figure S1).
3. Acetazolamide in Combination with Cisplatin and Fendiline Hydrochloride Reduces the Expression of Malignancy Markers of NB Xenografts In Vivo
To better characterize the mechanisms of the additive activity of acetazolamide on cisplatin/fendiline treatment, we analyzed the expression of specific markers of cell degeneration and malignancy in the SKNBE2 NB-generated subcutaneous nodules derived from all the above-described treatment groups.
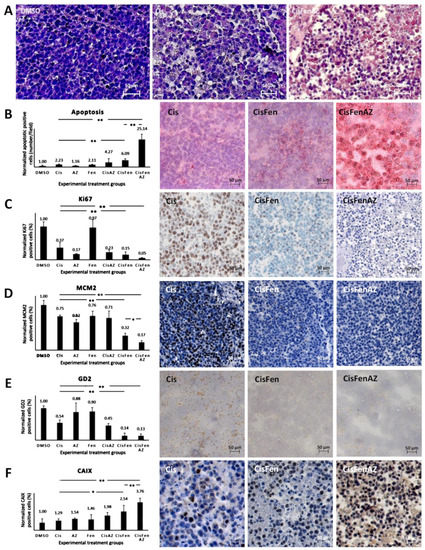
As shown by hematoxylin staining in Figure 2A, tumors from mice treated with Cis/Fen/Az showed several shrunken cells, with pyknotic nuclei and a less compact framework; nodule excision and manipulation were difficult as they were inconsistent, torn, disrupted, and prone to release a large quantity of dead cells. No viable cell culture was obtained from the disaggregation of the nodules.
Figure 2. Hystochemical analysis of SKNBE2 nodules. Representative images of hematoxylin staining of SKNBE2 nodules (A) from vehicle-(DMSO), cisplatin-(Cis) and cisplatin, fendiline, and acetazolamide-(CisFenAZ) treated mice. The percentage of pycnotic nuclei and mass disaggregation is strongly increased by CisFenAZ treatment. Quantification of apoptotic cells by TUNEL assay in SKNBE2 nodules after treatments (B). Values refer to the amount of TUNEL-positive cells per field (left plot). Panels on the right show representative images of cisplatin-(Cis), cisplatin/fendiline-(CisFen) and cisplatin/fendiline/acetazolamide-(CisFenAZ) treated mice. The number of apoptotic cells in cisplatin-treated tumors was increased by the addition of either acetazolamide (4.27 vs. 2.23) or fendiline (6.09 vs. 2.23); remarkably, the contemporary administration of acetazolamide and fendiline with cisplatin produced a strong increase of apoptotic cells (25.14 vs. 2.23). Analysis of Ki-67 (C), MCM2 (D), GD2 (E), and CAIX (F) expression in SKNBE2 nodules. Plots on the left quantify the amount of positive cells per field. Panels on the right show representative images of cisplatin-(Cis), cisplatin/fendiline-(Cis/Fen) and cisplatin/fendiline/acetazolamide-(CisFenAZ) treated mice. Cisplatin produced a significant reduction of Ki-67, MCM2, and GD2. The expression of Ki-67 was strongly reduced by acetazolamide alone and almost abolished when acetazolamide was added along with fendiline and cisplatin (C). MCM2 reduction by cisplatin was strengthened by fendiline and strengthened further by fendiline/acetazolamide (D). The effect of cisplatin on reducing GD2 was strengthened by fendiline only (E). All treatments except DMSO produced a significant increase in CAIX expression; the addition of acetazolamide induced a further highly significant CAIX increase, compared with CisFen (* p < 0.05, ** p < 0.001). Space bar—50 µM.
The toxicity of the treatments on tumor cells was initially highlighted (Figure 2B, right panels), and quantified (Figure 2B, left plot) by TUNEL assay, to detect apoptosis.
Results showed that: (1) treating the tumor with cisplatin increases the number of apoptotic cells in the nodules by 2.23-fold compared to the DMSO vehicle; (2) treatments with Cis/Az and Cis/Fen increase apoptosis by about 4.27 and 6.09-fold vis-à-vis controls, respectively; (3) the apoptotic rate in the nodules treated with Cis/Fen/Az increases by up to 25-fold. These data show that the addition of Az increases the cytotoxic activity of the cisplatin/fendiline combination by more than 4-fold, indicating an enhancement of the apoptosis rate induced by acetazolamide on the effect of Cis/Fen treatment.
The improved cisplatin/fendiline inhibition of nodule growth caused by acetazolamide (
Figure 1B) also suggests that a strong inhibition of cell proliferation may occur. Thus using immunohistochemistry we analyzed the relative percentage of cells of the excised nodules that expressed Ki67 and mini-chromosome maintenance protein 2 (MCM2), a protein involved in the initiation of genome replication now considered a sensitive proliferation marker in several types of human malignancy (
Figure 2C,D)
[9]. Cisplatin inhibited Ki67-expressing cells to 36% of the vehicle-treated control tumors, the addition of fendiline to cisplatin produced a further reduction to 15.17%, whereas the combination of Cis/Fen/Az leads to the almost complete suppression of expression with a further reduction of Ki67 positive cells (5.23% of DMSO controls) (
Figure 2C). Remarkably, the number of MCM2-positive cells was reduced with a similar pattern among treatments than observed for Ki67 (
Figure 2D), indicating that the combination of the three drugs is the most effective combination to counteract the proliferation of NB cells in the nodules, with a better outcome than cisplatin and Cis/Fen.
Next, we assessed whether the addition of acetazolamide to previous treatments induced the specific/preferential targeting of the most malignant cells. GD2 is a disialoganglioside antigen whose expression is associated with neuroectodermal origin tumor cells, and is considered a marker of NB malignant cells
[10]. We show that GD2-positive cells in sections from both Cis/Fen- and Cis/Fen/Az-treated mice were reduced to a similar level but the presence of these cells was significantly lower (p < 0.01) than in nodules from cisplatin-treated mice, and from DMSO-treated control animals (
Figure 2E).
Last, we analyzed the possible variation of the number of cells expressing CAIX in response to the inhibition of its activity by acetazolamide. We demonstrate that the synthesis of CAIX in the nodule cells increases along with the efficacy of the therapy, suggesting a likely response to the induction of hypoxia within the nodules caused by cytotoxic treatments. Additionally, in this case the treatment with Cis/Fen was more effective than that with Cis alone (2.53-fold vs. 1.29-fold increase), but the treatment with Cis/Fen/Az led to a 3.76-fold increase, emphasizing once again the effectiveness of acetazolamide as a component of the therapy (Figure 2F) (the statistical analysis of data is reported in Table S1).
4. Discussion
HR-NB causes about 15% of deaths from childhood cancers due to frequent relapses, metastasis, and resistance to chemotherapy
[11], which is likely to be dependent on stem-like cell populations
[12]. In particular, since HR-NB is poorly responsive to the currently available chemo- and radiotherapy protocols
[2], innovative treatment approaches are urgently needed. The conventional pharmacological approach to NB is based on cisplatin, either alone or in combination with other chemotherapeutics and radiotherapy
[13][14][15][16]. Nevertheless, a high level of resistance occurs through the stable hyperactivation of oncogenes, as mycN induces the expression of multidrug resistance genes
[17][18].
Increased knowledge of the molecular events leading to tumor growth, self-renewal, invasiveness, and drug resistance, has revealed a number of unexpected pathogens which can be targeted by new and old drugs, in repurposing approaches
[4][19]. Remarkably, several of these drugs have already been approved for human therapy, although not for anticancer purposes, and are well tolerated
[20]. As a consequence, their repositioning for anticancer therapies is a real opportunity to improve the efficacy of classical cytotoxic drugs. In this context, many studies have also shown the usefulness of drugs that are not directly cytotoxic but are able to counteract the self-defensive machinery of cancer cells, increasing the efficacy of classic chemotherapeutic agents
[21].
CAIX inhibition, by acetazolamide, a mild diuretic drug used for heart failure and glaucoma, or related compounds, was reported to inhibit neuroblastoma cell proliferation in conditions of hypoxia, and to increase the efficacy of chemotherapy
[22][23]. The additive activity of acetazolamide in combination with histone deacetylase inhibitor MS-275, reduced neuroblastoma cell growth and tumorigenicity, indicating that blocking CAIX activity can improve the outcome when used in combination with both classical and unconventional anticancer drugs
[24][25].
In conclusion, the present pilot study provides important proof of principle about the potential synergism of a combination therapy in which acetazolamide is added to fendiline and cisplatin, to increase chemotherapy cytotoxicity. Thus, the repurposing of this commonly used drug may increase the activity of anticancer drugs when conventional treatments are insufficient. However, although acetazolamide is generally well tolerated when administrated in different pathologies, possible side effects of its administration in this combination will need to be investigated in depth in studies analyzing higher number of mice and in more expanded preclinical studies.