1000/1000
Hot
Most Recent

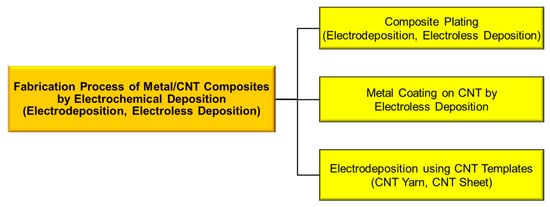
Metal/carbon nanotube (CNT) composites are promising functional materials due to the various superior properties of CNTs in addition to the characteristics of metals. Electrochemical deposition can be classified into three types: (1) composite plating by electrodeposition or electroless deposition, (2) metal coating on CNT by electroless deposition, and (3) electrodeposition using CNT templates, such as CNT sheets and CNT yarns.
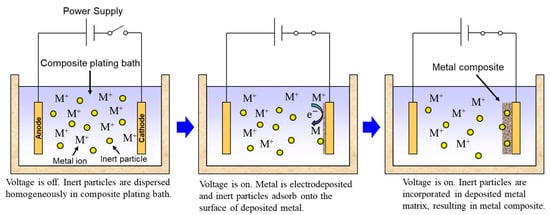
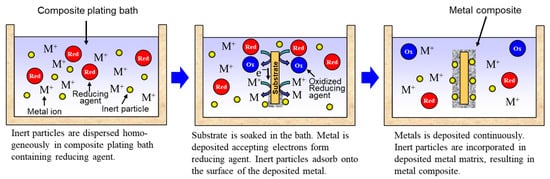
Rough schematics of composite plating by electrodeposition and electroless deposition are displayed in Figure 2 and Figure 3, respectively. In the case of electrodeposition, inert particles are dispersed homogeneously in a plating bath. When a voltage is applied, metal is electrodeposited on a cathode and the particles adsorb on the surface of the deposited metal. Then, the particles are embedded in depositing metal, resulting in a metal composite (Figure 2). In the case of CNT composite plating by electrodeposition, inert particles are dispersed homogeneously in a plating bath containing a reducing agent. When a substrate is soaked in the bath, metal is reductively deposited on the substrate accepting electrons from the reducing agent and, at the same time, the particles adsorb on the surface of the deposited metal. The particles are then embedded in depositing metal, resulting in a metal composite (Figure 3). In general, the substrate is pre-treated and catalyst particles, such as Pd particles, are fixed on the surface of the substrate before soaking into the plating bath. As far as was searched, the first article of the composite plating is on Cu/graphite composites by electrodeposition and was reported in 1928 [3]. Regarding the mechanism of the composite plating, several models have been proposed [4][5][6][7][8].
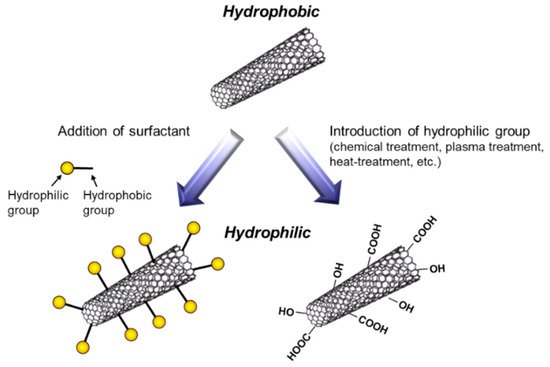
To fabricate metal/CNT composites with uniform distribution of CNTs, the preparation of plating baths with homogeneous dispersion of CNTs is important. In general, plating baths are aqueous solutions, while CNTs are hydrophobic. Therefore, hydrophilization of CNTs have been examined by the addition of surfactants or the direct introduction of hydrophilic groups on the surfaces of CNTs (Figure 4). The addition of surfactants in plating baths is a common method. Various kinds of surfactants [9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26], such as sodium dodecylbenzene sulfonate and sodium deoxycholate, have been examined for the homogeneous dispersion of CNTs in a pure water. However, effective surfactants for the dispersion in a pure water are not always effective in plating baths which contain great amounts of ions. Moreover, even if the surfactant is effective for the dispersion of CNTs in a plating bath, CNTs are not always co-deposited by electrochemical deposition. Therefore, the selection of appropriate surfactants is essential. Since the surfactants are likely incorporated in deposited metal matrix during electrochemical deposition, the concentration of surfactants should be examined. On the contrary, the direct introduction of hydrophilic groups, such as -COOH, onto the surfaces of CNTs has been examined using a chemical treatment [27], a plasma treatment [28], a heat treatment [29], and so on. These methods destroy the sp2 carbon bonding of the surfaces of CNTs. Therefore, the conditions of the treatments should be examined.
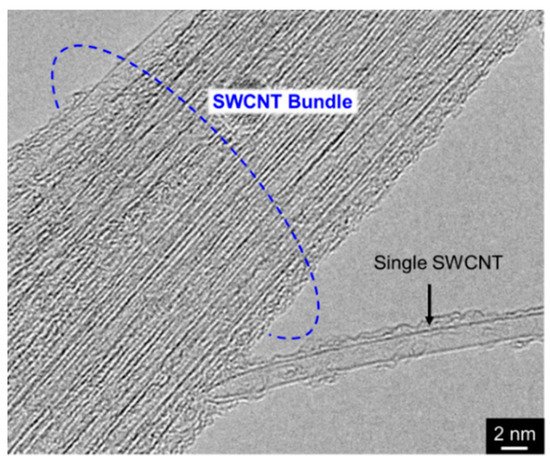
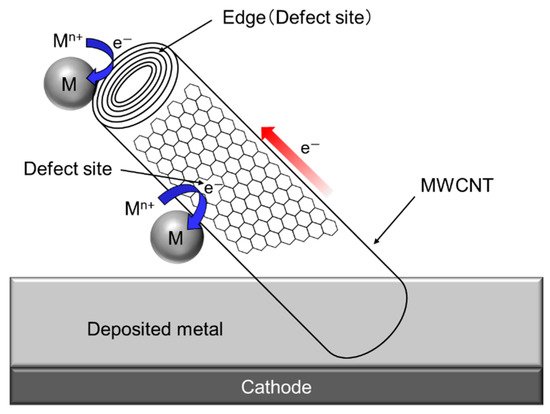
Since a single CNT, especially multi-walled CNT (MWCNT) has a fibrous shape with large aspect ratio in addition to a high electrical conductivity in the axis direction. Therefore, composite plating using CNTs as inert particles often shows a unique feature unlike other composite plating using insulation particles such as Al2O3 particles. The schematic of the unique feature is showed in Figure 6 [30]. When a part of a MWCNT is incorporated in the deposited metal matrix during electrodeposition, the metal can be electrodeposited not only on the deposited metal but also on the protruding edge (a defect site) of the MWCNT. If the defect sites exist on the sidewall of the MWCNT, the metal can also be electrodeposited on the defect sites.
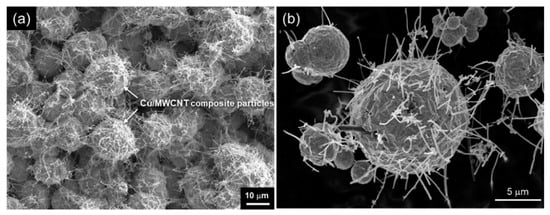
Fabrication conditions in these articles are listed in Table 1.
| Metal | CNT | Treatment of CNT | Base Plating Bath | Surfactant | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Sodium lauryl sulfate | Corrosion behavior | 2020 | [32] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Sodium lauryl sulfate | Corrosion protection | 2020 | [33] |
| Ni | MWCNT | Wrapped by polydopamine | Dull Watts bath | Non | Wear and corrosion resistance | 2019 | [34] |
| Ni | MWCNT | Non | Ionic liquid (choline chloride/carbamide) | Non | Non-aqueous solvent | 2017 | [35] |
| Ni | MWCNT | Non | Sulfamate bath | Cationic surfactant, compound name is unknown | Improvement in tool life | 2014 | [36] |
| Ni | MWCNT | Non | NiSO4+NaCl | Polyvinylpyrrolidone | Cyclic voltametric route | 2011 | [37] |
| Ni | MWCNT | Ball milling | Bright Watts bath | Sodium lauryl sulfate and Hydroxypropylcellulose |
Corrosion behavior | 2011 | [38] |
| Ni | MWCNT | Chemical treatment | Choline chloride/urea | Non | Non-aqueous solvent | 2010 | [39] |
| Ni | MWCNT | Non | Bright Watts bath | Polyacrylic acid | Solid lubrication | 2008 | [40] |
| Ni | MWCNT | Ball milling | Watts type bath | Sodium lauryl sulfate, Cetyltrimethylammonium bromide | Effects of surfactants | 2008 | [41] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Non | Effects of current density | 2008 | [42] |
| Ni | MWCNT | Ball milling | Bright Watts bath | Sodium lauryl sulfate and Hydroxypropylcellulose |
Mechanical properties | 2008 | [43] |
| Ni | MWCNT | Non | Bright Watts bath | Non | Mechanical properties | 2008 | [44] |
| Ni | MWCNT | Non | Bright Sulfamate bath | Polyacrylic acid | Low internal stress | 2007 | [45] |
| Ni | MWCNT | Non | Dull Watts bath | Non | Pulse-reverse parameter | 2007 | [46] |
| Ni | MWCNT | Non | Bright Watts bath | Polyacrylic acid | Thermal conductivity | 2006 | [47] |
| Ni | MWCNT | Non | Dull Watts bath | Poly(diallyldimethylammonium chrolide) | Pulse-reverse electrodeposition | 2005 | [48] |
| Ni | MWCNT | Chemical treatment | Dull Watts bath | Cetyltrimethylammonium bromide | Corrosion behavior | 2005 | [49] |
| Ni | MWCNT | Non | Dull Watts bath | Polyacrylic acid | Ni deposition on incorporated CNT | 2004 | [30] |
| Ni | MWCNT | Ball milling | Dull Watts bath | Non | CNT content | 2002 | [50] |
| Ni | MWCNT | Ball milling | Dull Watts bath | Non | Tribological property | 2001 | [51] |
| Ni-Co | MWCNT | Chemical treatment | Dull Watts bath + Co salt |
Non | Corrosion behavior | 2019 | [52] |
| Ni-P | MWCNT | Non | Dull Watts bath + citric acid + P compound | Polyacrylic acid | Tribological properties | 2010 | [53] |
| Ni-Co | MWCNT | Non | Dull Watts bath + Co salt | Compound name is unknown |
Mechanical and tribological properties | 2006 | [54] |
| Ni-P | MWCNT | Non | Ni salts + citric acid + P compounds | Compound name is unknown |
Corrosion properties | 2004 | [55] |
| Cu | MWCNT | Chemical treatment | Citric bath | Non | Corrosion behavior | 2021 | [56] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Pulse reverse, electrical conductivity | 2020 | [57] |
| Cu | MWCNT | Chemical treatment? | Sulfate bath | Non-ionic surfactants, Compound name is unknown | Mechanical properties, Microlaminated structure | 2020 | [58] |
| Cu | SWCNT | Non | Sulfate bath | Stearyltrimethylammonium chloride | Mechanical properties | 2020 | [59] |
| Cu | SWCNT | Non | Sulfate bath | Non | Microstructure | 2019 | [60] |
| Cu | MWCNT | Non | Sulfate bath | Sodium lauryl sulfate | Jet electrodeposition, Tribological properties |
2019 | [61] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Current collector for LIB anode | 2019 | [62] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Stearyltrimethylammonium bromide | Electrical conductivity, Corrosion resistance | 2018 | [63] |
| Cu | MWCNT | Non | Sulfate bath | Non-ionic surfactants, Compound name is unknown | Mechanical properties, Laminated structure | 2018 | [64] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2018 | [65] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2018 | [66] |
| Cu | MWCNT | Chemical treatment | Sulfate bath | Non | Cu/CNT powder + powder metallurgy | 2017 | [67] |
| Cu | MWCNT | Chemical treatment | Commercially available | Nano diamond | Periodic pulse reverse electrodeposition | 2016 | [68] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Current collector for LIB anode | 2016 | [69] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Co-deposition mechanism of CNT | 2013 | [70] |
| Cu | MWCNT | Non | Sulfate bath | Non | Electrochemical reduction behavior | 2011 | [71] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Pulse-reverse | 2011 | [72] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Surface morphology, Hardness, Internal stress | 2010 | [73] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Patterned field emitter | 2008 | [74] |
| Cu | SWCNT | Non | Sulfate bath | Commercial products | Mechanical properties | 2008 | [75] |
| Cu | SWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium chloride | Mechanical properties | 2008 | [76] |
| Cu | Cup-stacked CNT | Non | Sulfate bath | Polyacrylic acid | Various CNTs | 2005 | [77] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Microstructure | 2004 | [78] |
| Cu | MWCNT | Non | Sulfate bath | Polyacrylic acid | Cu/MWCNT composite powder | 2003 | [31] |
| Zn | MWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium bromide | Corrosion resistance | 2021 | [79] |
| Zn | MWCNT | Non | Zincate bath | Unknown | Pulse electrodeposition, Corrosion resistance | 2020 | [80] |
| Zn | MWCNT | Chemical treatment | Sulfate bath | Cetyltrimethylammonium bromide | Corrosion resistance | 2007 | [81] |
| Zn-Ni | MWCNT | Non | Chloride bath | Non | Pulse reverse, Tribological and Corrosion properties | 2016 | [82] |
| Cr | MWCNT | Non | Trivalent Cr bath | Sodium lauryl sulfate | Tribological properties, Corrosion resistance |
2020 | [83] |
| Cr | MWCNT | Non | Trivalent Cr bath | Sodium lauryl sulfate | Tribological properties | 2018 | [84] |
| Cr | MWCNT | Non | Trivalent Cr bath | Non | Mechanical properties | 2009 | [85] |
| Co | MWCNT | Non | Choline chloride/urea | Non | Non-aqueous solvent | 2017 | [86] |
| Co | MWCNT | Non | Sulfate bath | Polyacrylic acid | Field emission properties | 2013 | [87] |
| Co | MWCNT | Non | Sulfate bath | Polyacrylic acid | Tribological properties | 2013 | [88] |
| Co | MWCNT | Acid-treatment | Sulfate bath + citrate | Sodium lauryl sulfate | Tribological properties, Corrosion properties | 2013 | [89] |
| Co-W | MWCNT | Non | Co salt + Tungstate + Citrate | Polyacrylic acid | Tribological properties Corrosion properties |
2015 | [90] |
| Co-W | MWCNT | Non | Co salt + Tungstate + Citrate | Polyacrylic acid | Tribological properties | 2013 | [91] |
| Au | MWCNT | Non | Sulfite bath | Stearyltrimethylammonium chloride | Electrical conductivity, Tribological properties | 2009 | [92] |
| Ag | MWCNT | Non | Choline chloride + glycerol | Poly (N-vinyl pyrrolidone) | Pulse reverse electrodeposition | 2021 | [93] |
| Ag | MWCNT | Non | Iodide bath | Non | Electrical contact resistance against H2S gas | 2021 | [94] |
| Ag | MWCNT | Non | Iodide bath | Non | Hardness, Electrical and Tribological properties | 2020 | [95] |
| Ag | MWCNT | Non | Cyanide bath | Unknown | Electrical contact resistance against H2S gas | 2010 | [96] |
| Al | MWCNT | Acid treatment | Diethylene glycol dimethyl ether | Non | Hardness | 2020 | [97] |
| Al | MWCNT | Non | 1-ethyl-3-methylimidazolium chloride | Non | Hardness | 2006 | [98] |
| Sn | MWCNT | Non | Choline chloride + ethylene glycole | Non | Nucleation study | 2019 | [99] |
| Pb-Sn | MWCNT | Acid treatment | Fluoroborate bath | Polyacrylic acid | Corrosion resistance | 2010 | [100] |
Regarding the number of published articles on metal/CNT composite plating using electroless deposition, those on the Ni-P alloy/CNT is large. In the case of electroless deposition of Ni, phosphorous compounds such as sodium hypophosphite (NaH2PO2) are usually used as the reducing agent and the P derived from the NaH2PO2 is co-deposited with Ni, resulting in Ni-P alloy deposit. Most of the purpose of the fabrication of Ni-P alloy/CNT composites is the improvement of tribological properties. Fabrication conditions in these articles are listed in Table 2.
| Metal | CNT | Pre-Treatment of CNT | Reducing Agent | Surfactant | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ni-P | MWCNT | Non | NaH2PO2 | Sodium lauryl sulfate | Tribological properties, Corrosion resistance |
2021 | [101] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2012 | [102] |
| Ni-P | MWCNT | Ball milling, Chemical treatment |
NaH2PO2 | Commercial product | Tribological properties, Corrosion resistance |
2012 | [103] |
| Ni-P | MWCNT | Chemical treatment Ball milling | NaH2PO2 | Sodium lauryl sulfate | Mechanical attrition, Tribological properties | 2012 | [104] |
| Ni-P | MWCNT | HNO3 | Commercial product | Commercial product | Substrate: Mg powder | 2011 | [105] |
| Ni-P | MWCNT | Non | NaH2PO2 | Stearyltrimethylammonium chloride | Substrate: ABS resin Tribological properties |
2011 | [106] |
| Ni-P | MWCNT | Non | NaH2PO2 | Stearyltrimethylammonium chloride | Various P content, Tribological properties |
2010 | [107] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | Unknown | Effects on solder joint | 2009 | [108] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2009 | [109] |
| Ni-P | MWCNT | Chemical treatment | NaH2PO2 | unknown | Tribological properties | 2006 | [110] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Compound name is unknown | Hardness, Corrosion resistance |
2005 | [111] |
| Ni-P | SWCNT | Heat treatment | NaH2PO2 | Compound name is unknown | Tribological properties | 2004 | [112] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2003 | [113] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2003 | [114] |
| Ni-P | MWCNT | Ball milling | NaH2PO2 | Cetyltrimethylammonium bromide | Tribological properties | 2002 | [115] |
| Cu | SWCNT | Non | CHOCOOH | Sodium lauryl sulfate Hydroxypropylcellulose |
Mechanical disintegration, | 2016 | [116] |
| Cu | MWCNT | Non | CHOCOOH | Sodium lauryl sulfate Hydroxypropylcellulose |
Various CNTs Tribological properties |
2014 | [117] |
| Co-P | MWCNT | Non | NaH2PO2 | Non | Magnetic properties | 2016 | [118] |
A fabrication process of metal-coated CNTs by an autocatalytic electroless deposition is schematically showed in Figure 13. Even in the case of electroless deposition, homogeneous dispersion of CNTs in the plating bath is important. The introduction of functional groups on the surface of CNTs likely effective to increase deposition sites, resulting in CNTs coated by metal films and not metal particles.
Fabrication conditions in these articles are listed in Table 3.
| Metal | CNT | Pre-Treatment of CNT | Reducing Agent | Surfactant | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microstructure, Co-coated CNTs | 2020 | [119] |
| Ni-P | MWCNT | Introduction of -COOH on CNT + Pd2+ | NaH2PO2 | Non | EMI properties, Cotton fabric substrate | 2020 | [120] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Arc discharge synthesized CNTs | 2015 | [121] |
| Ni-P | MWCNT | Sn2+/Pd2+ commercial product | NaH2PO2 | Non | Fe-50Co composites, magnetic properties | 2014 | [122] |
| Au/Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Polyacrylic acid (Pre-treatment) |
Improved wettability with molten Al | 2012 | [123] |
| Fe-B/Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2, KBH4 | Non | Microwave absorbing properties | 2011 | [124] |
| Ni-P | SWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microstructure of Ni-layer | 2011 | [125] |
| Ni-B | MWCNT | Sn2+sensitization + Pd2+activation |
(CH3)2NH·BH3 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs Heat treatment |
2011 | [126] |
| Ni | MWCNT | Sn2+sensitization + Pd2+activation |
N2H4 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs Magnetic properties |
2010 | [127] |
| Ni-P | MWCNT | K2Cr2O7+H2SO4 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Microwave absorbing properties, Ni-N alloy | 2008 | [128] |
| Ni-P | MWCNT | HNO3 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Diallyl-dimethylammonium chloride | Graphitized MWCNTs | 2005 | [129] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Polyacrylic acid (Pre-treatment) |
Graphitized MWCNTs | 2004 | [130] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Continuous Ni-layer | 2002 | [131] |
| Ni-P | MWCNT | Mixed Pd2+/Sn2+ | NaH2PO2 | Non | Pd-coated CNTs | 1999 | [132] |
| Ni-P | MWCNT | Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Magnetic property | 1997 | [133] |
| Al | MWCNT | Sn2+/Pd2+ commercial product | LiAlH4 | Non | Non-aqueous bath: AlCl3-urea | 2020 | [134] |
| Ag | MWCNT | H2SO4 + HNO3 Sn2+sensitization + Pd2+activation |
HCHO | Non | Interfacial adhesion of composites | 2004 | [135] |
| Cu | MWCNT | Sulphoric acid + HNO3 Sn2+sensitization + Cu2+activation |
HCHO | Non | Electrical and mechanical properties | 2009 | [136] |
| Cu | MWCNT | HNO3 Sn2+sensitization + Pd2+activation HNO3 |
CHOCOOH | Diallyl-dimethylammonium chloride | Graphitized MWCNTs | 2004 | [137] |
| Co-P | MWCNT | K2Cr2O7+H2SO4 Sn2+sensitization + Pd2+activation |
NaH2PO2 | Non | Heat-treatment | 2000 | [138] |
CNT templates, such as CNT sheets [139][140][141][142] and CNT yarns or fibers [143][144][145][146], have been developed and their various practical applications have been researched. Although a single CNT has a high electrical conductivity, electrical conductivities of those templates are far less than metals such as Cu, due to the contact resistance between each CNT of which they consist. Therefore, metallization of the CNT templates is a promising process to give them enough electrical conductivity. On the contrary, CNTs have strong anisotropy in electrical and thermal properties [147]. Therefore, the orientation of CNTs which make up the templates is also important in order to achieve the expected properties of metal/CNT composites. Fabrication conditions in these articles are listed in Table 4.
| CNT Template | Feature of CNT Template |
Metal | Plating Bath | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|
| MWCNT film | Super-aligned | Cu, Ni | Acid sulfuric bath + glucose Dull Watts Bath |
Improved mechanical and electrical properties | 2019 | [148] |
| MWCNT film | Super-aligned | Ni | Dull Watts Bath | Improved mechanical properties | 2019 | [149] |
| SWCNT paper (Bucky paper) |
Orientation: in-plane direction | Cu | Acid sulfate bath + polyethylene glycol + Cl− + bis(3-sulfopropyl) disulfide + Janus green B | One-step electrodeposition by a combination of additives | 2017 | [150] |
| MWCNT paper | Super-aligned | Cu | Acid sulfuric bath + glucose + polyethylene glycol + Cl− Alkaline bath (EDTA, Citrate) |
Electrical conductivity | 2017 | [151] |
| MWCNT film | Super-aligned | Cu | Acid sulfuric bath + glucose | Improved mechanical properties | 2016 | [152] |
| MWCNT film | Super-aligned | Cu | Acid sulfuric bath + glucose | Improved mechanical properties | 2015 | [153] |
| SWCNT yarn | Straight | Cu | Acid sulfate bath | Graphen growth on the surface of electrodeposited Cu | 2021 | [154] |
| MWCNT yarn | Twisted | Cu | Acid sulfate bath + polyethylene glycol + Cl− + bis(3-sulfopropyl) disulfide + Janus green B | One-step electrodeposition by a combination of additives | 2020 | [155] |
| CNT yarn | Straight | Cu | Acid sulfate bath | Superior current carrying capacity | 2018 | [156] |
| MWCNT yarn | Twisted | Cu | (CH3COO)2 + CH3CN Acid sulfuric bath |
Effect of CNT yarn density | 2018 | [157] |
| MWCNT yarn | Twisted | Cu | Cu (CH3COO)2 + CH3CN Acid sulfuric bath |
Two-step electrodeposition Uniform composite wire |
2017 | [158] |
| MWCNT yarn | Twisted | Cu | (CH3COO)2 + CH3CN Acid sulfuric bath |
Two-step electrodeposition Electrical properties, Solderability, |
2017 | [159] |
| MWCNT yarn | Straight | Cu | Acid sulfuric bath | Electrodeposition of Cu interior of CNT yarn | 2016 | [160] |
| MWCNT yarn | Twisted | Ag, Pt | KNO3+AgNO3 H2SO4 + H2Pt6Cl6 |
Improved tensile strength and electrical conductivity | 2013 | [161] |
| MWCNT yarn | Twisted | Cu | Acid sulfuric bath + octyl phenyl poly (ethylene gylcol) ether | Continuous process: fiber spinning, anodization, electrodeposition | 2011 | [162] |
| MWCNT yarn | Twisted | Au, Pd, Pt, Cu, Ag, Ni | Metal salt solution | Self-fueled electrodeposition Improved electrical conductivity |
2010 | [163] |