The interaction of programmed cell death ligand-1 (PDL1) with its receptor PD1 inhibits T-cell responses. Blockade of this interaction with monoclonal antibodies leads to major antitumor effects. However, not all cancer patients respond well to anti-(PD-1/PD-L1) immunotherapy. The PD-L1 protein is expressed at the cell plasma membrane (mPD-L1), at the surface of exosomes (exoPD-L1), in cell nuclei (nPD-L1) and as a soluble circulating protein (sPD-L1). The aim of our analysis was to highlight the multiple variants of sPD-L1 generated either by the proteolytic cleavage of m/exoPD-L1 or by the alternative splicing of PD-L1 pre-mRNA. The objective was also to underline the presence and role of circulating sPD-L1 isoforms in multiple cancer indications and many other diseases (including chronic inflammatory and viral diseases), and under non-pathological conditions (pregnancy). sPD-L1 often represents a general marker of an inflammatory status. The pool of sPD-L1 proteins is an integral part of the highly dynamic PD-1/PD-L1 signaling pathway.
1. The PD-1/PD-L1 Checkpoint
The inhibitory checkpoint molecule programmed death-1 (PD-1) plays a vital role in maintaining immune homeostasis upon binding to its ligands PD-L1 and PD-L2. PD-1 (CD279) is an inhibitory receptor induced in activated T-cells and its two ligands are necessary to maintain peripheral tolerance. In addition, PD-L1 (CD274, B7-H1) plays a key role as a negative regulator of antitumor immunity, and the blockade of the PD-1/PD-L1 interaction permits one to restore and to increase the function of T-cells. Monoclonal antibodies directed against PD-1 (Nivolumab, Pembrolizumab, Cemiplimab, Spartalizumab, Camrelizumab, Sintilimab) or PD-L1 (Atezolizumab, Durvalumab, Avelumab, SHR-1316) are now used regularly for the treatment of multiple types of solid tumors, such as non-small cell lung cancer (NSCLC), melanoma, and gastric cancers to cite only a few types
[1]. Since the first approval of pembrolizumab in 2014 for the treatment of advanced or unresectable melanoma, seven antibodies targeting PD-1 or PD-L1 have been approved by the Food and Drug Administration (FDA) for the treatment of solid tumors or onco-hematological diseases (lymphoma)
[2]. These immune checkpoint inhibitors (ICI) have greatly improved patient survival in many advanced malignancies, but not all. Clinical responses are extremely variable from one cancer to another, and the durability of the response is also very variable from one patient to another. Spectacular responses have been observed in NSCLC and melanoma, whereas the response rate is much more limited in neuro-oncology, for example
[3]. Similarly, PD-1/PD-L1 checkpoint inhibitors are not very effective in treating acute myeloid leukemia
[4]. Only a small fraction of cancer patients respond well to PD-1/PD-L1 blockade. Combinations of chemo- or radiotherapy with immuno-therapy are largely developed to improve cancer treatment
[5][6].
1.1. Membrane PD-L1
PD-1 and PD-L1 are both membrane proteins expressed on immune cells and cancer cells, respectively. The success of an anti-PD-1/PD-L1 therapy greatly depends on the capacity of the injected antibody to activate T-cells to eliminate tumor cells, with the support of tumor-infiltrating lymphocytes. The membrane expression of PD-1 and PD-L1 is crucial to cancer immunotherapy, although in some cases there is little or no significant difference in overall survival between patients with PD-L1-positive and PD-L1-negative tumors
[7]. Expression of this immune checkpoints is not limited to T-cells and cancer cells. PD-1 is primarily expressed on the surface of T-cells, including regulatory T-cells (Treg), B cells, monocytes, dendritic cells (DC), and natural killer (NK) cells. Moreover, PD-1 expression can be induced by different cytokines (such as TGF-β and interleukin-10) on antigen-presenting cells (APCs), myeloid DC and monocytes, and under different pathological conditions such as preeclampsia, chronic viral infections, and cancer
[8]. On the other hand, PD-L1 is often highly expressed on tumor cells and can be present on activated T and B cells, DCs, monocytes and occasionally on endothelial and epithelial cells. Its expression can be induced by different inflammatory cytokines and interferon-γ (IFN-γ). The PD-1/PD-L1 checkpoint is associated with inflammatory effects, and it is now well established that the checkpoint plays a role in multiple diseases and conditions beyond cancer
[9]. Notably, the PD-1/PD-L1 checkpoint is largely implicated in reproductive immunology, with an important role in the establishment of immune tolerance mechanisms at the materno–fetal interface
[10].
In the oncology field, numerous studies have defined the potential values of PD-1/PD-L1 membrane expression as biomarkers for immunotherapy effectivity and tumor resistance in multiple cancer types. In general, a prominent PD-L1 expression on tumor cells and high levels of activated T-cells have been associated with a higher response rate. There is a satisfactory correlation between PD-L1 expression on tumoral cells and patients’ response to immune checkpoint inhibitors. The level of PD-L1 expression on circulating tumor cells (CTCs) is also considered a favorable biomarker in patients treated with ICIs, at least in the cases of NSCLC and melanoma
[11][12][13]. PD-L1 expressed on CTCs is also viewed as a useful biomarker for the early detection of cancers
[14]. However, it requires specific methods for CTC enrichment and the immune detection of PD-L1
+ CTC, using harmonized procedures
[15].
1.2. Exosomal PD-L1
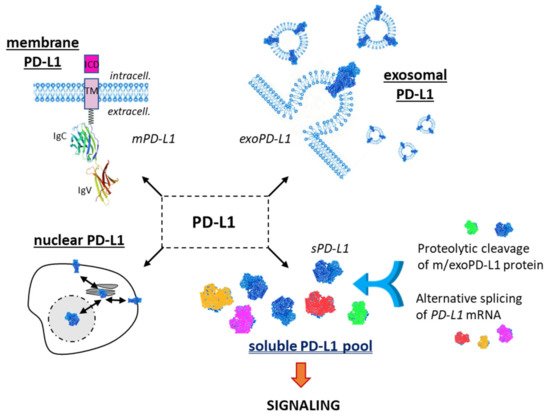
Besides the main form of PD-L1 expressed at the cell surface, notably on cancer cells, there are two other important forms of PD-L1 with distinctive roles (). PD-L1 can be expressed on cancer-derived exosomes, which are biologically active extracellular vesicles released from different types of tumor cells. These vesicles, with a cell membrane-like lipid-bilayer, can recapitulate the effect of cell-surface PD-L1 and they can significantly modulate the response to anti-PD-1/PD-L1 antibody therapy. They play a role in intercellular communications, in the composition and dynamic of the extracellular environment, and they can significantly affect the reactivity of the immune system
[16]. Exosomal PD-L1 (exoPD-L1) can be considered a mimic of tumor cell membrane PD-L1, capable of inhibiting T-cell immunity and enhancing the growth of various tumor types. Exosomal PD-L1 directly contributes to immunosuppression, not only in cancer
[17] but also in wound healing
[18]. There are several recent, comprehensive reviews about the biology and specific roles of exoPD-L1
[19][20][21][22][23][24]. Exosomal PD-L1 is a target similar to cell membrane PD-L1 (mPD-L1) and different approaches have been designed to reduce exosome biogenesis, secretion or to neutralize secreted exosomes, as recently discussed by Yin and coworkers
[25].
Figure 1. The different forms of the checkpoint protein PD-L1: membrane form (mPD-L1) with its characteristic transmembrane domain (TM) and intracellular domain (ICD); the exosomal form (exoPD-L1) embarked into extracellular vesicles released by a variety of cells; the nuclear form (nPD-L1) involved in the regulation of mRNA stability; the soluble forms (sPD-L1), which can derive either from proteolytic cleavage of m/exoPD-L1 or from an alternative mRNA splicing. m/exo/sPD-L1 can modulate T-lymphocyte activity via the PD-1 signaling pathway.
1.3. Soluble PD-L1
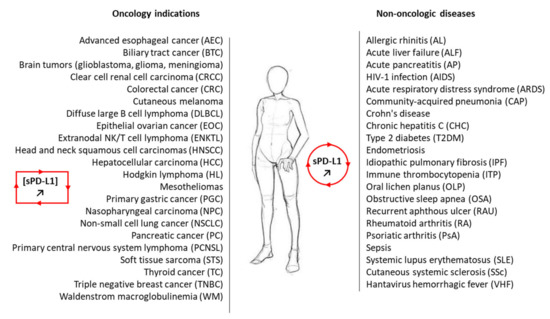
A third important PD-L1 entity is the soluble form, not anchored into a plasma membrane or a vesicle, but free in solution (). It is a circulating form, detected in the serum of cancer patients
[26], patients with auto-immune diseases or some viral diseases
[27][28], and other pathologies and conditions, including in pregnant women
[29]. sPD-L1 has been identified in more than 20 different pathologies and often plays an important immuno-regulatory role (). In cancer, both soluble forms of the checkpoint, soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1), have been detected in plasma, and elevated levels have been associated with advanced disease and worst prognosis for patients
[30]. There are different methods to detect and measure the level of sPD-L1 in the serum and plasma
[31][32][33][34]. Its role as a prognostic or predictive marker in lung cancer has been debated recently but the significance of this soluble protein remains uncertain
[35]. Here, we provide an update on the origin, biology and potential roles of sPD-L1 in different pathologies.
Our analysis deals with the soluble form of PD-L1, distinct from the exosomal form exoPD-L1, which is a membrane-bound form similar to mPD-L1. This point is essential because it requires robust methods to distinguish the free, soluble protein from the protein associated with extracellular vesicles (EV). Purification kits commonly used to isolate EV from biofluids, such as plasma or serum, may not always totally eliminate the free protein. Conversely, analysis of sPD-L1 in circulation using immune-assays (ELISA) may not distinguish between vesicular and soluble forms. This is a key aspect, perhaps insufficiently considered in some studies, although there are specific approaches to distinguish the two forms
[36][37].
The intracellular form of PD-L1 shall also not be neglected. It is a bioactive form, notably acting as an RNA-binding protein to regulate the mRNA stability
[38]. Nuclear PD-L1 is believed to play an important role in cancer cells independent of its function in immune checkpoint
[39][40]. However, for the sake of clarity, we will not discuss further the PD-L1 intracellular species here.
Figure 2. A non-exhaustive list of malignant and non-malignant diseases for which sPD-L1 has been measured in the plasma of patients. In most cases, the level of circulating sPD-L1 was found to be enhanced in the plasma of patients with the indicated pathology compared to healthy control (see . For the oncology indications, the presence of sPD-L1 and its significance are discussed in the following studies: AEC
[41]; BTC
[42][43]; brain tumors
[26][44]; CRCC
[45][46]; CRC
[47]; cutaneous melanoma
[48]; DLBCL
[49][50]; EOC
[51][52]; ENKTL
[53][54]; HNSCC
[36]; HCC
[55][56][57]; HL
[54][58]; mesothelioma
[59][60]; NC
[61][62]; PGC
[63][64][65]; NSCLC
[66][67]; PC
[68]; PCNSL
[50]; STS
[69][70]; TC
[71]; TNBC
[72]; WM
[73].
2. Significance of Soluble PD-L1 in Cancer
2.1. sPD-L1 as a Cancer Biomarker
Circulating soluble PD-L1 has been largely exploited as a diagnostic, therapeutic, or prognostic biomarker for cancers. The presence of sPD-L1, generated by the proteolytic cleavage of mPD-L1 and/or by the translation of alternatively spliced PD-L1 mRNA, has been confirmed in many types of cancers, such as those listed in
[74]. The molecule can be easily detected in the serum and plasma by various methods (mostly immuno assays (ELISA)) and its presence at a high level generally predicts a better response to an anti-PD-(L)1 mAb. More expression of sPD-L1 makes the response to anti-PD1 therapy more likely, in many cases (but not always). At the same time, a high level of soluble PD-L1 in peripheral blood often predicts poor prognosis in patients with solid tumors
[75]. However, as an isolated marker, it cannot be considered a predictor of overall survival of patients with solid tumors
[63]. The level of sPD-L1 does not always correlate with response to immune-sensitivity, notably in lung cancers
[45][66]. In some cases, the level of sPD-L1 is higher for metastatic patients compared to non-metastatic patients, as observed in clear cell renal cell carcinoma
[45], but again there is no general rule. The circulating levels of sPD-L1 represent a predictive biomarker of clinical response to anti-PD-L1 in mesothelioma patients
[59], and it might be a useful marker to predict the outcome in glioma patients receiving radiotherapy
[76]. Measurements of sPD-L1 could be useful to predict metastasis and prognosis in soft tissue sarcoma and hepatocellular carcinoma
[55][69]. An elevated serum level of sPD-L1 may also represent adverse prognostic factor in certain subtypes of T-cell lymphomas and leukemias
[77][78], but there is no general rule for all cancers. sPD-L1 levels vary enormously according to the lymphoma type. sPD-L1 levels are higher in patients with diffuse large B-cell lymphoma compared to healthy individuals but lower in patients with follicular lymphoma
[78]. In most of these studies, sPD-L1 is detected by ELISA, but the exact nature of the soluble protein (long form or truncated variants) is not determined. A better characterization of the circulating sPD-L1 form is recommended to better appreciate the potential role. We will not comment further on the biomarker aspect of sPD-L1, as there are specific recent reviews on this topic
[79][80][81][82][83][84][85].
The level of circulating sPD-L1 measured in the plasma or serum can vary considerably from one tumor type to another, and it can depend also on the stage of the disease, gender, and the associated treatment. Even in a defined cancer type and with a comparable method (ELISA), large differences of sPD-L1 levels have been reported, complicating the comparisons. For examples, in locally advanced non-small cell lung cancer (NSCLC), a median serum sPD-L1 concentration of 67–68 pg/mL has been reported
[66][86], whereas other studies indicated median sPD-L1 levels of 27 pg/mL
[87], 84 pg/mL
[88] and 176 pg/mL
[89]. Moreover, much higher values have been reported with other methods, up to 568 pg/mL measured using a multiplex assay
[67] and 3.84 ng/mL measured with another ELISA procedure
[90]. A quantitative comparison is thus difficult, if not impossible. Moreover, these measurements refer to the presence of sPD-L1 in the serum of patients, not the functionality of the circulating protein.
2.2. Functionality of sPD-L1 in Cancer
The question of the functionality of sPD-L1 in cancer is still disputed. Does sPD-L1 bind to PD-1? Yes. Does sPD-L1 deliver a positive or a negative regulatory signal through PD-1? The question remains open at present, although in most cases the answer to the question is “negative regulatory signal”. Different studies have indicated that sPD-L1 is a functional, glycosylated protein, capable of binding to PD-1
[91]. There are engineered variants of sPD-L1 (generated with directed molecular evolution) with an affinity over 20 folds greater than that of native human PD-L1 and able to compete with an anti-PD-1 antibody
[92]. In this study, native human sPD-L1 was found to induce suppressive effects on activated T-cells, thus mimicking the effect of mPD-L1
[93]. A recent study using sPD-L1 isolated from the plasma of patients with recurrent/metastatic breast cancer demonstrated that sPD-L1 can inhibit T lymphocyte function, acting as a negative regulatory element in cellular immunity
[94]. Similar observations have been reported previously in experimental (in vitro) studies using sPD-L1 released from lung cancer cells
[95]. sPD-L1 had been found to exert an immuno suppressive role either in inhibiting T-cell activation or promoting T-cell apoptosis
[96].
Similarly, sPD-1 also represents an active circulating protein, with an immune-modulatory capacity
[97]. Its activity can be exploited to combat cancer. Designed chimeric antigen receptor (CAR) T-cells which constitutively secrete sPD-1 have shown a marked antitumor activity
[98]. Proteolytic sPD-L1, notably the form released after the ADAM-cleavage of mPD-L1, is an active circulating protein capable of inducing apoptosis in CD
8+ T-cells and compromising the killing of tumor cells by these effector cells
[99]. Consequently, because it is an active signaling molecule, sPD-L1 can significantly influence the efficacy of therapeutic antibodies targeting the checkpoint. sPD-L1 could play the role of a sink for the injected PD-L1 inhibitors (antibodies or small molecules), acting as a decoy receptor for anti-PD-L1 antibodies, in the frame of a resistance mechanism
[100]. As a corollary, the therapeutic anti-PD-L1 entity can be useful to target simultaneously the different active species that are mPD-L1, exPD-L1 and sPD-L1. In particular, the targeting of sPD-L1 could be interesting to reinforce the antitumor effect of existing therapy (see below).
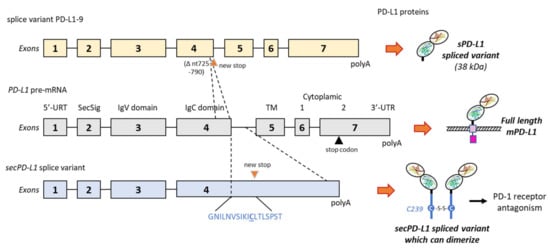
In most cases, sPD-L1 has been shown to function as a PD-1 blocker, as its parent product mPD-L1. However, a distinct situation has been reported with an sPD-L1 variant issued from alternative splicing, the above-mentioned form produced by exaptation of a LINE-2A intronic element. In this case, the specific sPD-L1 form lacked measurable T-cell inhibitory activity and, in sharp contrast, it was found to function as a PD-1 receptor antagonist, blocking the inhibitory activity of its competitors mPD-L1 and exPD-L1
[101]. In contrast, another study pointed out a specific splice variant producing a form of sPD-L1 (42-kDa) without the transmembrane domain but with a unique 18-amino acid tail (GNILNVSIKICLTLSPST) bearing a key cysteine (C) residue which allows the protein to homodimerize (). In this case, the splice form sPD-L1 was found to inhibit T-cell proliferation (without cell-to-cell interaction) and production of IFN-γ. It is important to underline that this form of sPD-L1 can be produced by many types of cancer cells (almost always in addition to mPD-L1) but also in normal cells
[102]. This specific sPD-L1 form is not exclusive to tumor cells, corroborating the detection of sPD-L1 in multiple other pathologies and conditions (see below). Different types of PD-L1 splice variants can be generated depending on the cells used and experimental conditions. For example, in melanoma cells (A375 and M34), up to four variants have been identified in addition to full-length PD-L1, with splices occurring from the exon 4 to exon 6, thus leading to the expression of various forms of sPD-L1. Three secreted forms of sPD-L1 (24, 38, and 45-kDa) have been detected in the supernatants of cultured melanoma cells. They were likely issued from the alternative splicing of the PD-L1 transcript, and they all have inhibitory functions on T-cell activation and proliferation
[48]. For example, the variant PD-L1-9 () has lost a 66-bp region in exon 4, inducing a frame shift leading to a stop codon before the transmembrane domain. In melanoma patients, three major splice variants of sPD-L1 originating from both tumor and immune cells, and differentially secreted, have been characterized. Their respective immunosuppressive role remains to be clarified
[48].
Figure 3. Two examples of alternative splicing of the PD-L1 pre-mRNA. The exons’ and introns’ organization of full-length PD-L1 is shown in the middle, and the full-length PD-L1 protein is shown on the right with its different domains (as in ). Above, the splice variant PD-L1-9, which has lost a 66-bp region from nt-725 to 790 in exon 4. The deletion indicates a frame shift leading to a stop codon before the transmembrane domain (TM). The variant produces a truncated sPD-L1 protein (38 kDa) lacking the TM and intracellular domains
[48]. Below is an alternatively spliced form of the human PD-L1 cDNA from placental tissue. The variant contains the first 4 exons of PD-L1, including the secretory signal (SecSig) at the N-terminus, IgV and IgC domains, which are shared with the full-length PD-L1. The variant does not splice into the fifth exon (encoding the transmembrane domain) but reads into the fourth intron, within a new stop codon. It produces an mRNA that lacks a transmembrane domain at its 3′ end and leads to a protein with the indicated unique C-terminal sequence. The underlined cysteine residue (C239) allows for protein homodimerization, as represented on the right side. The expressed protein, naturally dimerizing, was found to inhibit T-cell proliferation and production of IFN-γ from activated T-cells
[102].
Another immuno-active form of sPD-L1 has been identified in a large subset of cancer cell lines that express a high level of the PD-L1 gene with an exon-4 enrichment. Many cell types with a high expression level of exon 4 (e.g., RKO colon cancer cells; CAL62 thyroid anaplastic carcinoma cells) were found to express a truncated PD-L1 isoform which retained the ability to bind PD-1 and to function as a negative regulator of T-cell function, inhibiting IL-2 and IFN-γ secretion in primary T-cells
[103].
Therefore, according to the splicing process, an immuno-suppressive or a non-immuno-suppressive variant of sPD-L1 can be generated. The immuno-suppressive status may depend on the capacity of the secreted protein to dimerize, because PD-L1 dimerization is required to inhibit the activation of T lymphocytes. However, this hypothesis, entirely possible, remains to be validated. It is clear that there are multiple forms of circulating sPD-L1, cleaved forms generated by proteases and different alternative splicing variants. Altogether, they provide a structurally and functionally variable pool of soluble PD-L1 species.