Platelet-derived extracellular vesicles (pEVs) are nanosized membranous subcellular structures released by platelets, which comprise different subpopulations that differ on morphology, size, composition and cellular origin. Extracellular vesicles (EVs) work as intercellular communicators exerting their function by transporting their cargo that includes nucleic acids, proteins and lipids. pEVs have shown to mediate same functions as platelets, presenting a great potential for the development of new treatments in the biomedical field.
1. Introduction
In recent years, extracellular vesicles (EVs) have emerged as potential therapeutic effectors in the regenerative biomedical field. EVs are membranous subcellular structures released by any cell type, which comprise different subpopulations that differ on morphology, size, composition and cellular origin
[1]. In the past, EVs had been referred to by many different names such as microvesicles, exosomes, microparticles, apoptotic bodies, ectosomes or oncosomes, among others; according to their size, their tissue or cell origin, their claimed function or even their presence outside the cell
[2]. However, these EV subgroups presented a great diversity on the biomechanism behind their formation and the functions they perform, thus distinguishing them has not been proven to be easy
[3]. Therefore, a consensus has been reached and the most accepted classification is performed according to the characterization and the isolation methodology used
[1].
In general, EVs present a significant interest for the development of new treatments. EVs enable cell to cell communication, which can prevent the development of diseases by promoting homeostatic physiology or lead to pathological states, depending on the nature of the producing cell and the stimuli that activated the EV production
[4]. Different cellular mechanisms for EVs secretion and uptake exist, crucial for intercellular communication, which are still unknown
[5]. For this reason, some research focuses on the use of naturally produced EVs while other research aims to understand the molecular functionality of EVs to design new bioengineered carriers for enhanced cell delivery treatments or the addition of alternative cargos
[6][7].
Today, EVs are thought to be secreted by all cell types, being stem cells and immune cells, some of the most studied EV sources for therapeutical approaches
[8][9]. Nevertheless, clinical translation of cell cultured derived EVs has been hindered due to the high regulation requirements for ex vivo cell expansion
[10]. On the contrary, the use of platelets presents some advantages, mainly related to safety and regulatory concerns. On one hand, clinical-grade allogenic platelets can be obtained from whole-blood donations as a byproduct from red blood cells obtention. On the other hand, compared to other cell sources, ex vivo cell expansion is avoided, and the human origin and the lack of growth medium components diminishes concerns over contamination or immunological safety
[10]. Thereby, although relatively little attention has been paid so far to the therapeutic use of platelet EVs (pEVs), platelets and its concentrates are emerging as a potential source that overcome the limitations of other EV sources for regenerative medicine.
Platelet concentrates, such as platelet rich plasma (PRP) or platelet lysate (PL), are biological samples that have already been widely evaluated in regenerative medicine
[11]. Thus, the use of platelet concentrates in regenerative approaches has already been reviewed elsewhere
[12][13]. Some of the main fields in which platelet concentrates are being used include dermatology, aesthetic medicine, musculoskeletal regeneration, cardiovascular diseases, or neural regeneration among others
[14]. The therapeutical applicability of PRP was first associated to the biomolecules released by platelets, mainly attributed to growth factors. In fact, platelets can release growth factors, cytokines and extracellular matrix modulators that promote revascularization, restoration of damaged tissue and activation of mesenchymal stem cells
[15][16]. However, it has not been until relatively recently that pEVs have also emerged as a potential effector of platelet concentrates and platelets themselves, involved in their regenerative and therapeutical application
[17].
While until recently the use of pEVs in therapeutics have not been explored, already in 1967, Peter Wolf described the release by platelets of minute lipid-rich particulate material, which could be separated by ultracentrifugation, distinguishable from intact platelets and showing coagulant properties, terming this minute particulate as “platelet dust”
[18]. Later on, future studies identified platelet-released particles again, which were observed in electron microscopy samples. This further characterization and description allowed a renaming of “platelet dust” for a more accurate term: microparticles
[19]. Further on, the particle release was observed in many other cell types, thus microparticles were joined up with what are now called EVs
[1]. The aggrupation the different historical names under the common label of EVs aims to lead to a more comprehensive and accurate report of the activity and functionality of EVs bringing consensus among the different disciplines
[1].
After the initial studies performed on the functionality of “platelet dust” or platelet microparticles, it seems now clear that pEVs appear to be important effectors not only for coagulation but also for platelet regenerative function along with the rest of the biomolecules released by platelets
[10].
Even more, the clinical use of platelet concentrates is still limited due to its main drawbacks that are the lack of reproducibility, mainly due to the non-standardized separation methods, the variability among donors or the storage conditions
[20][21][22]. Moreover, the use of autologous concentrates limits the total obtained volume and needs programming of its obtention to arrange a proper treatment
[15][23]. Even more, some patients may not be suited for this sort of interventions due to their medical record, e.g., cancer patients or tobacco users
[24]. Together, along with the lack of quality controls, this leads to high heterogeneity of the obtained concentrates. Therefore, pEVs are a promising alternative to surpass PRP and other platelet concentrates limitations
[11], due to providing off-the-shelf controlled product methods
[25][26].
On the other hand, pEVs may surpass the platelet concentrate limitations and even present some desirable advantages that could improve the benefits of their clinical use. For instance, not only do pEVs share platelet function but they are more powerful, in terms of coagulation
[27] or osteogenic
[25][26] capacity. In addition, pEVs in contrast to platelets, can cross tissue barriers, extending their abilities beyond the blood
[17]. In fact, pEVs have been identified in some spatial contexts where platelets are rarely found, such as the synovial fluid, the lymph or the bone marrow, and like other types of EVs they are expected to be able to cross other tissue barriers including the blood-brain barrier
[17].
2. Regenerative Effects of pEVs
Recently, pEVs have been postulated to play a key role in homeostatic processes
[28]. In fact, platelets and pEVs are natural mediators of different physiological processes and contribute to the immune system response functions and regenerative process
[29]. However, only a few articles have evaluated the potential of pEVs as therapeutic regenerative tools (
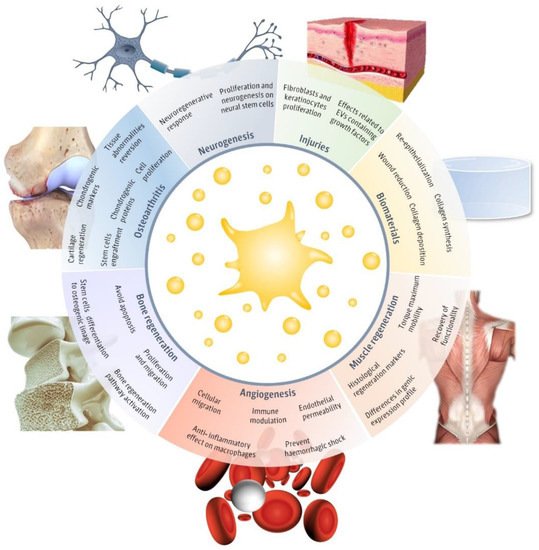
Figure 1). The main fields in which pEVs have been evaluated include injuries, neurogenesis, muscle regeneration, angiogenesis, biomaterials, bone regeneration, and osteoarthritis. Therefore, in this review, we aim to detail the main advances in the different regenerative approaches evaluated until now in order to assemble their applicability and realize of the main limitations that still hinder pEVs clinical use.
Figure 1. Regenerative applications of platelet-derived extracellular vesicles (pEVs). Main regenerative effects reported for pEVs in regenerative fields, including injuries
[30][31][32][33][34][35][36], biomaterials
[30][31], neurogenesis
[37][38], muscle regeneration
[39], angiogenesis
[37][38][40][41][42], bone regeneration
[25][26][43][44][45] and osteoarthritis
[46][47][48][49] and the major reported therapeutical effects. This figure was created using Freepik images.
One of the main fields in which the applications of pEVs have been studied are injuries and wounds. Concretely, an increase of fibroblast and keratinocyte migration and proliferation in vitro has been reported, associated with the wound healing process
[30][32]. These effects may be related to the pEVs cargo, which was positive in different growth factors, including platelet-derived growth factor (PDGF), basic fibroblasts growth factors (FGF2), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF)
[30]. Even more, the evaluation on a diabetic rat model confirms in vivo the wound regenerative effects observed for pEVs
[30][31]. In the same direction, more creative experiments suggest that pEVs can be combined with biomaterials or active biomolecules to obtain improved regenerative results. Interestingly, pEVs were combined with a sodium alginate hydrogel in order to achieve a more translational medical product, despite reaching similar properties than using pEVs directly
[30]. Another study presented pEVs formulated on a chitosan/silk hydrogel and combined this approach with a plant polysaccharide. This study reports higher collagen synthesis and deposition, wound reduction, re-epithelialization, and dermal angiogenesis in vivo
[31]. It is suggested that angiogenesis induced by pEVs may be mediated through Erk and Akt pathways, while reepithelization is triggered by the activation of yes-associated protein (YAP)
[30].
Furthermore, in addition to the wound healing properties, two rat model studies suggest that pEVs prevent uncontrolled blood loss and hemorrhagic shock
[33][34][35]. In fact, the pEVs dose-response performed in vitro suggests that pEV blood coagulation is dependent on EVs concentration
[33], as the International Society for Extracellular Vesicles (ISEV) encourages to test
[1]. Even more, pEVs have an effect on endothelial permeability, which mitigates blood loss too
[34]. Further studies report that aggregates of thrombin activated pEVs decrease the bleeding time after in vivo injuries while decreasing the interleukin concentration too
[35]. Interestingly, pEVs have been used after being stored at −20 °C, proving to maintain the positive effects for hemorrhagic shock treatment and easing their use
[33], thus being an attractive alternative to liquid platelet-rich plasma preparations that need to be kept at temperatures of 20–24 °C and with a short half-life (approximately 5 days)
[34][50].
Moreover, it is important to realize that pEVs are also involved in the inflammatory response. Some studies report that pEVs present an anti-inflammatory effect on stimulated macrophages, which decreased the release of cytokines, such as the tumor necrosis factor alpha (TNF-α) or interleukin 10 (IL-10)
[36]. Even more, non-therapeutical studies have reported that pEVs may act as inflammation modulators, inducing pro-inflammatory or anti-inflammatory responses depending on the stimuli conditions
[17]. However, few studies have been performed to date on evaluating pEVs treatment effects on immune modulation, although the pEV role is known to be involved in the inflammation processes
[28]. In addition, it is important to note that pEVs may be conditioned to the storage time of PRP until its use. In fact, pEVs have shown that, during platelet incubation, plasma proteins can be incorporated to pEVs altering their composition
[36].
Another interesting property of pEVs treatments is their angiogenic capability, associated with cellular mobilization and migration. In fact, vasoregeneration and maintenance of arterial integrity after injury have been reported by different studies
[40][41][42]. These effects were attributed to pEVs protein cargo, such as PDGF, FGF2, and VEGF, and also to lipid growth factors, despite not being directly identified
[41][42]. Incorporation of pEVs into cells and later phenotypical changes were assessed through in vitro studies
[40]. Later in vitro and in vivo experiments confirmed an increase in cell recruitment and adhesion, followed by a regenerative effect
[40]. Even more, rat ischemic hearts were analyzed in vivo confirming the angiogenic effects of pEVs
[42]. A dose-dependent angiogenic effect has been reported for pEVs
[41].
In more specific studies, pEVs have also been reported to be involved in the neuroregenerative response. First, in vitro studies suggest that pEVs induce proliferation and neurogenesis on neural stem cells, which have been associated with different proteins contained in pEVs, such as PDGF, FGF2, and VEGF
[37]. Even more, the use of pEVs induces higher increase on Erk and Akt pathways than the direct treatment with these growth factors alone
[37]. Secondly, in vivo studies show an increase in neural stem cells proliferation and differentiation, in addition to the angiogenic effect. Furthermore, the rat model evaluated improved the neurological functionality after ischemic stroke according to a motor disability test
[38]. Overall, it is interesting to notice that the neuroregenerative effects attributed to pEVs follow a dose-dependent response, as it has been reported
[37][38].
Another field in which pEVs have been evaluated as therapeutical agents is musculoskeletal regeneration. To start, it has been suggested that pEVs may contain a functional miRNA profile that would benefit osteoarthritis regenerative therapies
[46]. Chondrocyte cell culture studies have shown that pEVs induce an increase on proliferation and cell migration through the activation of the Wnt/β-catenin signaling pathway
[47]. Moreover, pEV treated chondrocytes have shown a decrease in the proinflammatory response and the apoptosis rate induced by inflammation conditions
[47][48]. As a functionality test, pEV treatment promoted the expression of chondrogenic markers on patient derived osteoarthritic chondrocytes
[48]. Moreover, the pEVs effects also induced a decrease in the proinflammatory profile of chondrocytes, suggesting an improvement for osteoarthritis treatment reflected on cellular morphology and protein expression
[48]. Furthermore, these effects observed for pEVs follow a dose-dependent response
[47]. The functional effects were corroborated in an in vivo approach, in which an osteoarthritic rabbit model was used. In this study, higher levels of chondrogenic proteins were found for the pEV treated group, while the tissular abnormalities observed in the histological cuts were reversed
[47]. Finally, pEVs have also been evaluated in combination with other approaches such as cell therapy. Specifically, pEVs enhance the engraftment of stem cells into articular injured tissue, thus promoting the cartilage regeneration in intra-articular defects
[49].
In bone regeneration, in silico evaluation of pEV miRNA also suggested their potential use for bone repair
[43]. These predictions have been supported for some in vitro studies, which report that pEVs promote the differentiation of mesenchymal stromal cells into the osteogenic linage
[25][26]. It was shown that pEVs can be internalized by stem cells and, after 20 h, they were mainly colocalized in the perinuclear region. Moreover, pEVs induced proliferation and migration of stem cells in a dose-dependent manner
[26]. Osteo-differentiation effects in vitro were determined by calcium determination by Alizarin red staining
[26] and the expression of cellular osteogenic markers
[25]. The osteogenic effects in vitro have been attributed not only to the growth factors pEVs contained, like VEGF, PDGF, FGF2, or TGFβ, but also to their genetic material, such as RNA
[26][43]. In addition, in vitro and in vivo models of osteonecrosis have been used to test pEV functionality. These models suggest that pEVs can promote proliferation and avoid apoptosis, inducing a bone regeneration effect through the activation of Akt/Bad/Bcl-2 pathway
[44]. However, another study performed in pigs had previously reported no significant effects in bone formation, despite having induced angiogenesis in the pEVs treated group
[45]. Therefore, it is necessary to perform further experiments, and proper pEVs characterization, to determine their real osteogenic effect.
Finally, pEVs are also associated with muscle regeneration. pEVs induced an increase of histological regeneration markers, such as centrally nucleated fibers, after an in vivo rat study. Even more, pEVs treated group showed an improved recovery of functionality, associated with the torque maximum mobility
[39]. Furthermore, this study compared the gene expression profile of inflammatory, fibrotic, and regenerative related markers of pEVs and stem cell-derived EVs. This comparison allowed to see differences on the gene expression despite similar functional regenerative outcomes
[39].