The degradation of substrates with a high protein content results in a high level of ammonium (NH
4+-N) in the residue, which causes process instability and reduced biogas/methane production
[5]. At a NH
4+-N concentration above 3 g/L, acetate degradation changes from acetotrophic methanogenesis to syntrophic acetate oxidation
[6][7]. The syntrophic co-operation between syntrophic acetate-oxidizing (SAO) bacteria and hydrotrophic methanogens means that the oxidation of acetate is combined with hydrogen consumption. Cocultures of SAO bacteria and methanogens usually have a lower growth rate than acetic methanogens, which decreases biogas production.
Another ingredient influencing anaerobic digestion is sulfuric acid, which is used to regulate the pH during alcohol production. This contributes to the high concentration of sulfates in distillery stillage
[8]. Sulfate-reducing bacteria convert sulfates into sulfides
[9]. Degradation of amino acids releases sulfides from the amino acids, methionine and cysteine, which results in the release of organic sulfur in the form of sulfides. Sulfides cause corrosion, decrease the bioavailability of trace elements essential for microbial activity by forming complexes with metals, and inhibit the growth of micro-organisms and the consumption of organic compounds (mainly alcohols, volatile fatty acids (VFAs)) and hydrogen, which could be used for biogas production
[10][11].
2.1. Bioreactors and Operational Parameters
Conventional anaerobic digestion systems require a long hydraulic retention time (HRT) of approximately 30–40 days, which means that they need to be modified to prevent biomass from leaching from the reactors.
Distillery stillage has been treated in various types of anaerobic digesters, including a sequencing batch reactor (ASBR), an up-flow anaerobic sludge blanket reactor (UASB), an anaerobic continuous stirred tank reactor (CSTR), an anaerobic baffled reactor (ABR), a down-flow stationary fixed film (DSFF), and an anaerobic membrane bioreactor (AnMBR), with COD removal ranging from 82 to 99% and an organic loading rate (OLR) ranging from 2.9 to 29 kg COD/(m
3·d)
[13][14][15][16][17][18][19][20].
As an example, the ASBR allowed a soluble COD removal efficiency greater than 98% to be obtained at an OLR of 8.6 kg COD/(m
3·d) at an HRT of 2.2 days
[13]. Melamane et al.
[21] reported the application of the down-flow fluidization technology for the anaerobic digestion of distillery stillage, in which 85% total organic carbon (TOC) removal was achieved at an OLR of 4.5 kg TOC/(m
3·d). However, UASB reactors are the most used high-rate digesters for anaerobic treatment of various types of industrial wastewaters. In a UASB treating distillery wastewater, COD removal efficiency of over 90% was reported
[22]. In another anaerobic treatment method, fluidized bed reactors contained appropriate media, such as sand, gravel, or plastics, for bacterial attachment and growth. A two-stage process with an anaerobic filter, followed by a UASB reactor was investigated by Blonskaja et al.
[23]. The acidogenic and methanogenic phases were separated, ensuring better conditions for the methanogens. COD removal was 54 and 93% in these stages, respectively. In general, such two-stage systems were found to enable better conditions for the methanogenic phase, thus being more suitable for anaerobic digestion of distillery waste
[24].
In the conventional CSTR systems, the purpose of anaerobic digestion is to maintain the stable process conditions, along with shortening the HRT, because this allows conversion of a higher amount of distillery stillage to energy. Lee et al.
[14] examined the shortening HRT in a CSTR during the anaerobic digestion of distillery stillage obtained at corn ethanol production. The results showed no differences in volatile solid (VS) reduction (82–83%) in the reactor, with HRTs ranging from 25 to 40 days. The maximum rate of the methane production of 1.41 L CH
4/(L·d) was produced at 25-day HRT, whereas the maximum methane yield of approximately 0.63 L CH
4/g VS was achieved at HRTs between 30 and 40 days. Simulation results using a kinetic model indicated that the reactor needs to be operated for longer than 23 days to achieve 80% of the maximum methane yield.
The increase in process efficiency in the conventional CSTRs can be achieved by increasing the temperature, which also leads to shortening the HRT. Anaerobic digestion of corn ethanol thin stillage was tested at thermophilic temperature (55 °C) in two CSTRs. The thin stillage was organically concentrated with 100 g COD
tot/L and 60 g VS/L and a low pH of approximately 4.0. Steady-state conditions were achieved at 30-, 20-, and 15-day HRTs, and digester failure was obtained at a 12-day HRT. A significant reduction in VS was achieved, with a maximum reduction (89.8%) at the 20-day HRT. Methane yield ranged from 0.6 to 0.7 L CH
4/g VS removed during steady-state operation. Effluent VFAs below 200 mg/L as acetic acid were achieved at 20- and 30-day HRTs
[15]. Oosterkamp et al.
[16] managed to shorten HRT to 10 days treating distillery stillage in the CSTR also under thermophilic (55 °C) conditions. The methane production was 0.43 L CH
4/(g COD·d), COD removal was 64%, soluble COD removal was 62%, and pH was 7.7.
Operating at a high OLR (>25 kg COD/(m
3·d)) and short HRT (less than 5 days) is possible when the micro-organisms are retained inside the reactor. Andalib et al.
[17] tested the anaerobic fluidized bed bioreactor (AFBR) employing zeolite with an average diameter (dm) of 425–610 μm and a specific surface area of the carrier media of 26.5 m
2/g. Despite a very high concentration of distillery stillage with COD of 130 g COD/L and total suspended solids (TSS) of 47 g/L, the AFBR showed up to 88% COD and 78% TSS removal at a very high OLR of 29 kg COD/(m
3·d) and HRT of 3.5 days. Methane production rates of up to 160 L/d at the steady-state equivalent to 40 L CH
4/L distillery stillage and biogas production rate per reactor volume of 15.8 L/(L·d) were achieved.
The reduction in biomass washout, higher solid retention time (SRT), and significantly improved phase separation can be achieved in the hybrid configuration of an anaerobic baffled reactor (ABR) where solid/liquid/gas separators were incorporated into the configuration of the conventional ABR
[18]. The hybrid ABR achieved higher COD removal, sulfate removal, and methane yield of 97–94%, 94–97%, and 294–310 mL CH
4/g COD, respectively, at an OLR of 1.0–3.5 kg COD/(m
3·d) than conventional ABR, where 75–94% COD removal, 67–76% sulfate removal, and 140–240 mL CH
4/g COD were obtained at an OLR range of 1.1–1.8 kg COD/(m
3·d).
Thin stillage from a dry-grind corn ethanol plant was evaluated as a carbon source for anaerobic digestion by batch and high-rate semi-continuous down-flow stationary fixed film (DSFF) reactors. Continuous studies employed two mesophilic DSFF anaerobic digesters treating thin stillage operated at HRTs of 20.0, 14.3, 8.7, 6.3, 5.0, and 4.2 d. Successful digestion was achieved up to an OLR of approximately 7.4 g COD/(L·d) at an HRT of 5 d, with a yield of 2.05 L CH
4/(L·d) and COD
tot, and VS removal efficiencies of 89% and 85%, respectively
[19].
Besides the operational conditions, lipids in the lipid-rich distillery stillage cause operational problems in anaerobic digesters due to clogging and mass transfer problems because they are adsorbed to the microbial biomass surface. The flotation of biomass due to adhesion of fat may cause loss of active biomass because of washout. An excellent solution to biomass washout problems was reported for the treatment of lipid-rich wastewater in granular sludge bed reactors. The potential of anaerobic membrane bioreactors (AnMBRs) for the treatment of lipid-rich corn-to-ethanol thin stillage was investigated by Dereli et al.
[20] at three different SRTs of 20, 30, and 50 days. The AnMBRs achieved up to 99% COD removal efficiencies and excellent effluent quality. Although higher OLRs up to 8.0 kg COD/(m
3·d) could be applied in the reactors operated at shorter SRTs, better biological degradation efficiencies, i.e., up to 83%, were achieved at increased SRTs. Severe long-chain fatty acid (LCFA) inhibition was observed at 50-day SRT, possibly caused by the extensive dissolution of LCFA in the reactor, inhibiting the methanogenic biomass.
Maintaining stable anaerobic digestion, despite the rapid acidification and accumulation of intermediate VFAs lowering microbial activity and biomethane production, can be challenging. Therefore, more research is focused on how to recover anaerobic digestion performance after acidic shock (pH 5.5). The carbonaceous materials may function as an abiotic conductive conduit to stimulate microbial electron transfer and resist adverse effects on anaerobic digestion. Wu et al.
[25] tested the nanomaterial graphene and more cost-effective pyrochar in their ability to recover anaerobic digestion performance after acidic shock (pH 5.5). Results showed that graphene addition (1.0 g/L) could lead to a biomethane yield of 250 mL/g COD; this was an 11% increase compared to the control. The recovered process was accompanied by faster propionate degradation (CH
3CH
2COO
− + 2H
2O
→ CH
3COO
− + CO
2 + 6H
+ + 6e
−). The enhanced performance was possibly ascribed to the high electrical conductivity of graphene. In comparison, pyrochar addition (1 and 10 g/L) did not enhance the biomethane yield, though it reduced the digestion lag-phase time by 18.1 and 12.2% compared to the control, respectively. Microbial taxonomy analysis suggested that
Methanosarcina (81.5% in abundance) with diverse metabolic pathways and OTU in the order DTU014 (6.4% in abundance) might participate in direct interspecies electron transfer, contributing to an effective recovery from acidic shock.
2.2. Effect of Polyphenols and Melanoidin on Biomethane Production
It was reported that distillery waste contains recalcitrant compounds, namely polyphenols and melanoidins, which exhibit toxicity towards micro-organisms
[26][27][28]. Although the concentrations of polyphenols in some distillery wastes (molasses distillery wastewater) are more than two times lower
[29] and they contribute less to the antimicrobial effect, at the same concentrations, polyphenols have a higher antimicrobial effect than melanoidins
[26].
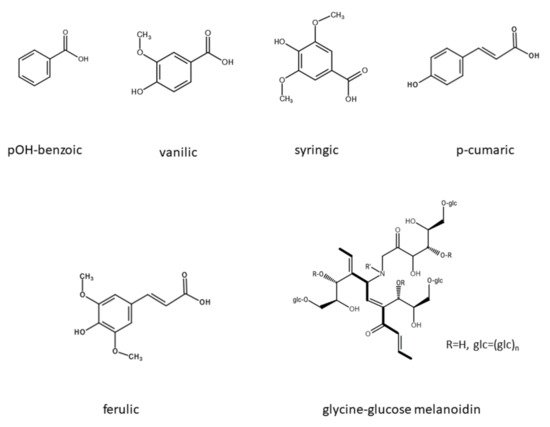
Figure 1 shows the structure of common polyphenols and melanoidins that are found in the distillery stillage.
Polyphenols are the compounds that particularly adversely affect methanogenesis, thus inhibiting the ability of methanogens to produce biofuels. According to Fedorak and Hrudey
[30], this inhibition is visible at polyphenol concentrations above 1 g/L. The concentrations of phenolic compounds above 1 g/L decreased the biogas production from waste by 10%, and concentrations increased to 1.5, 2.0, and 4.0 g/L reduced the production by 29, 78, and 98%, respectively
[31]. In other studies on anaerobic digestion, biogas production was decreased by phenol concentrations above 0.8 g/L
[32]. On the other hand, at concentrations 120–594 mg/L, biogas production was decreased by up to 50%, depending on the polarity, type, molecular size, and amount of the phenolic compounds
[33]. Melanoidins account for over 2% (m/v) of molasses distillery wastewater composition
[34]. Although the toxicity of melanoidins is lower, under anaerobic conditions, its brown color is intensified
[35], which impedes decolorization of the effluents.
Figure 1. The structure of polyphenols and melanoidin (based on
[36]) typical for the distillery stillage.
To improve biomethane production and to ensure the profitability of biogas plants, technologies for accelerating biomethane production from wastes that contain polyphenols should be developed. The antioxidative properties of polyphenols and melanoidins made them important for food, cosmetics, and pharmaceutical applications. Therefore, their recovery from distillery waste can bring two advantages: obtaining products of commercial interest and improving anaerobic digestion of this waste. For example, Kaushik et al.
[37] used ultrafiltration, adsorption–desorption, and solvent extraction for recovering polyphenols and melanoidins from sugarcane molasses distillery wastewater. Methane content in produced biogas increased from 51% in the control sample to 70% after adsorption, which improved the methanation the most from the tested options. From molasses distillery effluents, melanoidins were removed with biological and physicochemical methods, as well as employing microbial fuel cells for electricity generation
[38]. Biological methods included the use of a pure bacterial consortium comprising
Proteus mirabilis,
Bacillus sp.,
Raoultella planticola, and
Enterobacter sakazakii [34], or phycoremediation
[12]. Physicochemical methods included electrochemical degradation performed with ruthenium-oxide-coated titanium mesh (anode) and stainless steel (cathode)
[39], or UV photodegradation
[40].
2.4. Post-Treatment of Distillery Stillage
To valorize distillery wastes, anaerobic digestion is mostly used to recover energy in the form of biogas. However, the effluent from anaerobic digestion still contains organic compounds and is dark in color. As an example, anaerobically digested stillage may contain 25,000–40,000 mg COD/L, 7000–10,000 mg BOD/L, and 22,000–34,000 mg TSS/L
[12]. It was found that anaerobic processing does not decrease polyphenol content
[26]. In addition, some polyphenols may be transformed from one form to another during different phases of the fermentation process
[45], and, also, the transformation of polyphenols to less colored but more toxic products can proceed during the treatment of distillery waste with fungus
Pleurotus sp. under aerobic conditions
[46]. Therefore, to meet the environmental discharge standards, further treatment is necessary. Most often, when the BOD/COD ratio of the anaerobic effluent is greater than 0.25, the effluent is treated aerobically.
In the development of biological methods, the ability of some microbial strains or consortia to biodegrade anaerobically digested distillery stillage was examined. Efficient biodegradation was obtained when using such bacterial genera as
Pseudomonas,
Bacillus,
Microbacterium,
Achromobacter,
Staphylococcus, and
Alcaligenes [47][48]. According to Mohana et al.
[49],
Pseudomonas,
Stenotrophomonas, and
Proteus were able to remove COD (51%) and color (67%) within 72 h. The decolorization was possible by excretion of manganese peroxidase and laccase that removed the melanoidin by over 70%
[34]. Removal of color was also achieved when employing yeast strains for the post-treatment of molasses distillery wastewater
[50]; the removal efficiencies of color, COD, and BOD by
Citeromyces sp. were 75, almost 100, and 76%, respectively. From the same type of wastewater, extracellular enzymes secreted by white-rot fungi (manganese peroxidase, lignin peroxidase, and phenol oxidase (laccase)) allowed efficient removal of melanoidin and polyphenols
[51].
Apart from pure cultures, mixed cultures of activated sludge were reported to efficiently remove tannic acid polyphenol-containing wastewater under aerobic conditions at dissolved oxygen concentrations above 1 mg/L
[52]. To enhance microbial growth and improve polyphenol degradation, supplementation of carbon sources was carried out
[53]. The studies of aerobic treatment of anaerobically digested distillery stillage focused on the optimization of the operating parameters, particularly OLR. In the sequencing batch reactor (SBR) operated at a constant HRT of 24 h, an increase in OLR from 1.8 to 9 kg COD/(m
3·d) decreased the SBR performance
[54]. The highest removal of COD (74%) and BOD (96%) was obtained at 3.6 kg COD/(m
3·d). Apart from activated sludge reactors, biofilm in a rotating biological contactor was used after treating distillery stillage in microbial fuel cells
[55]. The removal of COD and BOD was 84 and 81%, respectively.
The combination of activated sludge treatment with a biomass separation on a membrane in the technology of membrane bioreactors (MBR) results in a recovery of high-quality effluent when treating distillery stillage, a smaller footprint, and reduced sludge generation. In a lab-scale MBR, 95% COD reduction and 92% decolorization were achieved
[56]. At an OLR between 3.0 and 5.7 kg COD/(m
3·d), 41% COD removal
[57] or 60% COD removal
[58] was obtained, depending on the cut-off of the membrane used. In the study by Deschamps et al.
[59], the membrane was directly incorporated into the anaerobic treatment, which produced a pilot-scale AnMBR. High biogas production of 1.36 NL
biogas/(L
bioreactor·d) was obtained at an OLR of 3.97 kg COD/(m
3·d) and HRT of 3.5 d. By comparison, with the anaerobic packed-bed bioreactor, it was found that the higher COD removal efficiency (96.9%) and higher methane production (0.26 L CH
4/g COD) were obtained in the AnMBR with a shorter start-up period (21 d).
The limitations of aerobic processes for post-treatment of distillery stillage include the energy costs for aeration, high amount of excess sludge produced, necessity of nutrient supplementation, and operation at high dilution rates
[26]. To make the post-treatment more energetically efficient, phycoremediation using microalgae was applied
[12]. Particularly, effluents from the acidogenic process can be used by these photosynthetic organisms, also termed photobiocapture organisms. In this process, microalgae use nutrients present in the effluent and sunlight for growth. Moreover, carbon dioxide is absorbed by microalgae and converted into oxygen, thus reducing the energy requirements. Biomass produced may be further utilized for the production of biogas, biodiesel, or fertilizer. However, the feasibility of this technology depends on sufficient sunlight accessibility in a particular location. As an example, 83.2% of COD and 88.0% of BOD were removed in microalgae ponds with an HRT of 11 d
[60].
For post-treatment, physicochemical methods are also used; however, they are more effective for low-loaded effluents than biological processes. To recycle the water, reverse osmosis allowed for recovering 60% of water, with a permeate COD of 100 mg/L
[12]. However, a high-pressure drop on the membrane under the conditions of high pollutant load and the necessity to utilize retentate increase the total operational cost.
The other solution that allows for water recovery from distillery thin stillage is evaporation that increases the solid content to 55–60%
[61]. Burning stillage generates steam, which can be used as evaporation fuel, to generate electricity or run a turbine. The condensate from the evaporation system can be recycled back to the fermentation process. However, the process is highly energy-intensive; 550 kcal of energy is required to evaporate 1 L of water
[12].
The other post-treatment process used is ultraviolet (UV) photodegradation. In the study by Apollo et al.
[40], it was found that UV photodegradation is effective in color removal, but not effective in COD and BOD removal from distillery effluent. On the other hand, anaerobic digestion alone removed COD effectively, but the color was not sufficiently removed, or even increased because of the conversion of color imparting compounds, such as melanoidin. Therefore, using UV photodegradation as a post-treatment to the anaerobic digestion allowed COD removal of above 85% and 88% of color removal to be obtained.