In recent years, new targets have been included between the health outcomes induced by pesticide exposure. The gastrointestinal tract is a key physical and biological barrier and it represents a primary site of exposure to toxic agents. Recently, the intestinal microbiota has emerged as a notable factor regulating pesticides’ toxicity. However, the specific mechanisms related to this interaction are not well known.
1. Introduction
In recent years, the demand for food has risen significantly in relation to the world population’s increase. At present, different types of pesticides are widely used worldwide to obtain better quality agricultural products and increase crop yields, thus bringing significant economic benefits. Pesticides reach the soil, water and air, and non-target organisms, including humans
[1]. As a result, health concerns about pesticide risks to animals have increased in recent years
[2][3][4]. A growing number of studies have reported that pesticides are linked to various pathologies, including metabolic diseases (such as obesity and type 2 diabetes)
[5], dysregulation of the immune system
[6][7][8], neurotoxicity
[9], endocrine alterations, reproductive disorders
[10][11], and even tumours
[12][13], whereas the gastrointestinal microbiota critically contributes to a variety of host metabolic and immune functions.
Many efforts, focusing on the mechanisms of oxidative stress and the role played by the genetic profile in terms of susceptibility, have been conducted
[14][15][16]. Recently, different studies have demonstrated that gut microbiota (GM) can be affected and altered by different types of environmental pollutants, including some pesticides
[17], therefore the role of the gut microbiota in pesticide-induced toxicity in non-target organisms is gaining increasing attention
[18].
The GM refers to the complex set of gut-resident bacteria, fungi and viruses that have mutualistic relationships with their host. About 10–100 trillion symbiotic microbial cells live in the human intestine
[19]; the intestinal microbiota’s main components are bacteria species taxonomically classified by genus, family, order, and phyla.
[20]. The GM is complex and challenging to characterize. The intestinal bacterial community can be mainly divided into 2172 species isolated in human beings, classified into 12 different phyla, of which 93.5% belong to
Proteobacteria, Firmicutes, Actinobacteria and
Bacteroidetes [21]. The microbiota composition and diversity remain relatively stable throughout life, although some changes have been observed in infancy and old age
[22]. Several scientific studies point out that maintaining a healthy GM plays a key role in human health. The intestinal microbiota acts as an interface between foods, allows the assimilation of nutrients, helps the digestion of fibres, participates in the synthesis of some vitamins and amino acids, and regulates the absorption of fatty acids, calcium and magnesium. Furthermore, the gut microbiota is indispensable for the development and maturation of the gastrointestinal tract
[23][24], a protective barrier against pathogenic microorganisms and toxins. The loss of stability of a healthy microbiota leads to the transition from eubiosis to dysbiosis and, consequently, pathological conditions ranging from inflammatory damage to chronic degenerative diseases, including tumours and neurological disorders
[25][26][27].
In vitro, in vivo and epidemiological studies have linked human exposure to pesticides to several chronic diseases. In vivo, these effects have been investigated primarily in model organisms representing humans (such as rats or mice).
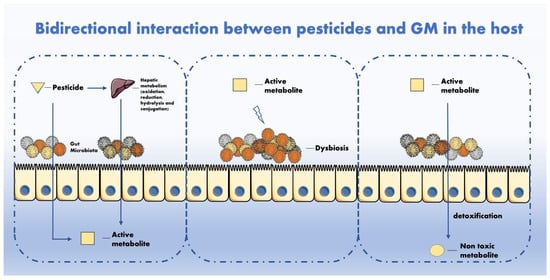
The mechanisms of interaction between pesticides and GM have been hypothesized in other studies
[28][29] and will therefore be described shortly in this manuscript. Regarding how pesticides can interact with GM, the main types of interactions are illustrated in . The GM metabolizes multiple chemicals after intake. Mutually, pesticides can impair the GM activity and composition with adverse repercussions for the host. Though it is known that GM perturbations can affect the homeostatic equilibrium between favourable and hostile microorganisms, it is not still clear how the GM and pesticides interact and whether these interactions are relevant for human health. In fact, it is reasonable to affirm that not all statistically significant perturbations are also biologically relevant and vice versa
[30].
Figure 1. GM metabolizes multiple chemicals after intake. Mutually, pesticides can impair GM activity and composition with adverse repercussion for the host.
2. Organophosphate Pesticides (OPPs)
2.1. Chlorpyrifos
Chlorpyrifos (CPF) represents one of the most used organophosphorus pesticides. Many recent studies have investigated the effect of this toxicant in animal models, mainly rats and mice. Chronic exposure seems not only to affect the gut but also influence serum gonadotropins levels (follicle-stimulating hormone, luteinizing hormone and testosterone) and raise proinflammatory cytokines (such as IL-6, monocyte chemoattractant protein-1, and TNF-α) in rats, suggesting a potential role in the development of infertility and colitis
[31]. Rats born from an exposed mother to oral CPF administration were smaller and lighter than non-exposed. Moreover, gut histological structures and the microbial community were altered; intestinal villi were shorter and thinner, and probiotic bacteria resulted as being lower in the ileum, caecum and colon
[24]. It has been hypothesized that CPF could modify the gut microbiota composition in a diet-specific manner
[17], even if authors observed differences only at the taxonomic level of genus and species but not at phylum
[32]. Long-term exposure to CPF in mice could raise gut absorbency, reducing the gene expression of tight junction proteins such as occludin, claudin 1, and ZO-1, both in ileum and colon. It was observed that exposed mice gut microbiota showed an increment in
Proteobacteria phylum and a decrement in
Bacteroidetes phylum, suggesting that these phyla are mainly affected by CPF exposure
[33]. Still, long-term exposure seems to amplify spontaneous vertical activity and female activity after acute stress in Wistar rats
[34]. The host’s genetic profile can change the gut microbial response to CPF exposure. Guardia-Escote et al. orally exposed mice to CPF apoE3 and apoE4, observing differences between the two genotypes at different taxonomic levels; the phylum
Verrucomicrobia resulted as highly represented only in apoE4 mice
[35]. Subsequently to a daily dose of 1 mg/kg for 30 days in mice, besides altered gut microbiota, CPF also induced the alteration of urine metabolites, amino acids and short-chain fatty acids (SCFAs). As a result, the GM abundance of
Firmicutes resulted as weakened, unlike
Bacteroidetes, which resulted as higher
[36]. Gut microbiota is supposed to be responsible for pesticide resistance
[37]. A model organism silkworm, ‘
Bombyx mori’, was fed with different antibiotics to induce gut dysbiosis and then exposed to CPF. It was observed that the antibiotic-fed silkworm larvae showed a significant amount of
Firmicutes and
Actinobacteria but a minor amount of
Proteobacteria. Additionally, the antibiotic-treated silkworms seemed to be more vulnerable to CPF, supporting the hypothesis that the gut microbiota plays a crucial role in pesticide resistance
[38]. Though, other authors did not demonstrate a shift in a mosquito gut microbial community, despite the pesticide-degrading microbiota resulting enriched
[39]. In a
Drosophila melanogaster model,
Lactobacillus plantarum (
Firmicutes) showed a greater capacity compared to
Acetobacter indonesiensis (
Proteobacteria), in the metabolization of CPF to the more effective metabolite chlorpyrifos oxon (CPO)
[40].
Other authors highlighted CPF effects on oxidative stress and gut microbiota dysbiosis in zebrafish. They found an abundance of
Proteobacteria with an alteration of
α-Proteobacteria,
β-
Proteobacteria, and
γ-
Proteobacteria in CPF-treated zebrafishes compared to the control
[41]. Still, through the use of an in vitro culture system, the gut microbiota of a marine benthic polychaete, ‘
Nereis succinea’, showed fundamental metabolizing activity towards two representative organophosphates, CPF and malathion
[42]. The impact of CPF on the gut microbiota has also been studied in vitro using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME
®) and Caco-2/TC7 cells of the intestinal mucosa. After 3.5 mg/day CPF exposure,
Lactobacillus (
Firmicutes) and
Bifidobacterium (
Actinobacteria) concentrations resulted as being strongly decreased
[43]. A low-dose exposure (1 mg/day) after 15 and 30 days increased the
Enterobacteria spp.
(Proteobacteria),
Bacteroides spp. (
Bacteroidetes) and
Clostridia (
Firmicutes) counts but decreased the
bifidobacterial (Actinobacteria) counts, reducing fermentative efficacy
[44]. Similar results were obtained from other authors who aimed to assess the effect of CPF chronic low-dose exposure (1 mg for 30 days), both on SHIME and rats. They observed an increment in the entire bacterial flora, primarily caused by
Enterococcus spp. (
Firmicutes) and
Bacteroides spp. (
Bacteroidetes), but a reduction in
bifidobacterial (
Actinobacteria) and
lactobacilli (
Firmicutes)
[45].
2.2. Trichlorfon, Diazinon, Monocrotophos, Dichlorvos, Phoxim
The effects of trichlorfon administered to common carp at different concentrations (0, 0.1, 0.5 and 1.0 mg/L) were evaluated by Chang et al. Gut histological changes resulted in a reduced size of intestinal villus and an altered expression of tight junction proteins, such as claudin-2, occludin and ZO-1. In addition, many antioxidant enzymes were decreased and proinflammatory cytokines upregulated after treatment. The most represented phyla were
Fusobacteria,
Proteobacteria and
Bacteroidetes. Trichlorfon administration led to a reduction in
Fusobacteria and an increment in
Bacteroidetes [46]. Inversely, diethyl phosphate exposure did not show proinflammatory effects in exposed rats. Opportunistic pathogens were found significantly enriched, but IL-6 was reduced
[47]. The effects of another organophosphorus pesticide, diazinon, were evaluated in crucian carp. Diazinon exposure increased the counts of
Bacteroidetes, Fusobacteria and
Firmicutes, but not the abundance of
Proteobacteria. After 14 days of exposure, also
Patescibacteria were found to be incremented
[48]. Low monocrotophos exposure led to a depletion of the bacterial and fungal populations in the earthworm gut (
Lampito mauritii). After 15 days of exposure, the bacteria and fungi showed a minor increment, but on day 30, the total counts were lower than those of the control earthworms
[49]. It has been hypothesized that gut microbiota could modulate resistance to dichlorvos in a species of beetles,
Callosobruchus maculatus [50]; a similar mechanism has been suggested to explain the resistance of some strains of
Anopheles mosquitoes to organophosphate fenitrothion
[51]. Phoxim exposure in silkworms showed immunosuppressive effects and increased bacterial abundance, particularly in two genera, such as
Methylobacterium and
Aurantimonadaceae (
Proteobacteria)
[52]. See .
3. Organochlorine Pesticides (OCPs)
p, p’-dichlorodiphenyldichloroethylene (p, p’-DDE), Dieldrin, Endosulfan, Indoxacarb, Hexachlorocyclohexane, Malachite Green
Dichlorodiphenyldichloroethylene (DDE) is the most prevalent metabolite of dichlorodiphenyltrichloroethane (DDT), a common organochloride pesticide banned several decades ago. Among all the adverse effects of organochlorides, the GM is also a target of these chemicals. In mice exposed for 8 weeks to DDE, alterations of gut microbiota composition resulted in an increment in
Bacteroidetes, and a reduction in
Proteobacteria,
Deferribacteres and
Cyanobacteria [53]. Contrariwise, in rats exposed to DDE, gut dysbiosis resulted in a reduction in
Bacteroidetes and
Proteobacteria accompanied by increased
Firmicutes and
Tenericutes, with a substantial rise in the
Firmicutes-to-
Bacteroidetes ratio
[54]. Other authors investigated the effects of both DDE and β-hexachlorocyclohexane (β-HCH), an active metabolite of hexachlorocyclohexane (HCH), on mice, observing changes in hepatic and bile acid metabolism besides the aforementioned effects on the microbiota composition
[55]. HCH, being a persistent organic pollutant, can alter the microbial composition in the human colostrum and, consequently, the colonization of the infant gut
[56]. These results were confirmed by another study that investigated whether environmental pollutants, including organochlorine, in breastmilk could impact the homeostasis of the infant gut microbiota at one month, revealing differences in the abundance of some strains of
Firmicutes [57]. Zebrafish fed with dieldrin did not show histological modifications of the gastrointestinal tract or body weight changes after four months of exposure. However,
Firmicutes resulted as less abundant
[58].
Malachite green (MG) (an organic chloride salt used as a fungicide in the aquaculture industry) has been shown to influence not only gut microbiota but also immune system homeostasis in goldfish. The altered gut microbiota was linked to opportunistic bacteria overgrowth and thus to potential infections
[59]. Other authors observed a diet-specific effect of endosulfan sulphate, a major metabolite of the organochlorine insecticide endosulfan, in mice. The abundance of most the represented phyla (such as
Actinobacteria,
Proteobacteria,
Bacteroidetes and
Firmicutes) was different in the mice fed with a high-fat diet from low-fat diet fed mice
[60]. The GM’s role against insecticide resistance has been demonstrated in the German cockroach. Indeed, using antibiotic treatments, the susceptibility to indoxacarb insecticide was incremented. The main phyla composing microbiota were
Proteobacteria,
Bacteroidetes,
Firmicutes and
Fusobacteria, and via faecal transplant, indoxacarb-resistant could be transferred to susceptible strains
[61]. See .
4. Insecticides
4.1. Neonicotinoid, Imidacloprid
The toxicity of imidacloprid (IMI), a neonicotinoid insecticide, has received growing attention in the last years. In a
Drosophila melanogaster model, IMI led to microbial dysregulation consisting of an increment in
Lactobacillus spp.
(Firmicutes) and
Acetobacter spp.
(Proteobacteria) [62]. Bees can be an indirect target of neonicotinoid insecticides such as IMI, thiacloprid, nitenpyram, amitraz and dimethoate. Several studies suggested that long-term exposure can lead to impairment of the gut microbial in bees with a reduced abundance of total bacterial flora, mainly
Firmicutes [63][64][65][66][67]. In pregnant mice, nitenpyram also altered lipid metabolism and decreased the abundance of
Proteobacteria, specifically of the Desulfovibrionaceae family
[68], and IMI was shown to reduce liver weights and alter the histological structure of hepatic tissue, disrupting the gut microbial composition with consequently impaired barrier function
[69]. Hong et al. evaluated the effects of IMI in crab, ‘
Erocheir sinensis’. On the one hand,
Bacteroidetes abundance was increased in a dose-dependent manner and reached almost three times as much as the control. On the other hand, the relative abundance of
Proteobacteria diminished
[70]. Onaru et al. described the effects of clothianidin on rats. The animals showed a loss of body weight and significant alterations in the gut microbiota, principally
Firmicutes was strongly decreased, inversely
Bacteroidetes was augmented and the dominance of
Proteobacteria and
Actinobacteria was inverted
[71].
4.2. Pyrethroid
Among all pyrethroids, permethrin (PEM) is one of the most commonly used for pest control. In low-dose exposed rats, PEM decreased the amount of three microbial species belonging to the
Bacteroidetes phylum (
Bacteroides,
Prevotella and
Porphyromonas). Moreover, it showed antibacterial properties against beneficial bacteria such as
Bifidobacterium (
Actinobacteria) and
Lactobacillus (
Firmicutes)
[72]. The widespread use of pyrethroids, such as alpha-cypermethrin, deltamethrin and PEM, in the ecosystem could modify the bacterial composition in mosquitoes, promoting insecticide-metabolizing microbiota, thus supporting insecticide resistance
[73]. Further, methoprene, a biochemical insecticide, was capable of affecting the microbiota in larval mosquitoes, arising the abundances of Proteobacteria, and precisely the Gammaproteobacteria class and Enterobacteriaceae family
[74]. In vitro analysis of intestinal epithelial cells confirms that gut microbiota activity can be disrupted by deltamethrin
[18]. The gut microbial community in wasps has been suggested to determine animals’ susceptibility to insecticides such as abamectin
[75]. Rouzé et al. investigated the effect of four pesticides (IMI, coumaphos, fipronil and thiamethoxam) on the GM of honeybees, highlighting how the microbial community’s composition differed in summer and winter bees. Sublethal doses of the compounds mentioned above showed similar effects and these were more substantial in the winter bees
[76]. Other authors focused on the toxicity of aldicarb, a carbamate insecticide, on mice models, demonstrating through a metabolomic approach how this chemical can disrupt the gut microbiota leading to alteration in lipid profile, oxidative stress induction, DNA damage and susceptibility to bacterial pathogenicity
[77]. See .
5. Fungicides
Fungicides aim to guard crops against parasitic fungi or spores. One of the most common fungicides is benzimidazole carbendazim (CBZ). It has been demonstrated that a 14-week exposure in mice induced changes in gut microbiota composition and an increment in body weight. Specifically, the amounts of
Bacteroidetes and
Verrucomicrobia were seriously lower,
Actinobacteria increased significantly, while
Firmicutes and
Proteobacteria had no remarkable modification
[78]. An additional study conducted on mice demonstrated that the bioaccumulation of CBZ was lower in the liver and gut compared to the faeces, suggesting a strong interaction with the gut microbial community until excretion. The faecal analysis showed a strong decrement in
Bacteroidetes after five days of exposure; conversely, the relative abundance of
Firmicutes,
Proteobacteria and
Actinobacteria increased
[79]. The abundance of
Firmicutes,
Bacteroidetes,
Actinobacteria,
Proteobacteria and
Verrucomicrobia were decreased in the gut of zebrafish exposed to CBZ for 21 days
[80]. Otherwise, difenoconazole led to an increment in
Firmicutes,
Proteobacteria (
Aeromonas and
Enterobacteriaceae) and
Bacteroidetes (
Bacteroides)
[81]. Xu et al. evaluated the chronic toxicity induced by epoxiconazole in rats. After 90 days of exposure, the richness of
Firmicutes diminished while
Bacteroidetes and
Proteobacteria grew
[82]. The mice exposed to penconazole showed a reduced amount of
Proteobacteria, while
Bacteroidetes,
Cyanobacteria and
Actinobacteria significantly increased. Both enantiomers toxicity was assessed and revealed substantial alteration in the abundances of gut microbial communities
[83]. The fungicide azoxystrobin has proved able to alter the GM in soil earthworms, ‘Enchytraeus crypticus’, specifically
Proteobacteria, which significantly increased after the 2 and 5 mg/kg exposure
[84]. After 15 weeks of exposure to 2.5 mg/kg imazalil (IMZ), the microbial communities in the caecum and faeces of mice were significantly altered at the phylum and genus levels, with a substantial decline in
Bacteroidetes,
Firmicutes and
Actinobacteria [85]. Previously, the same authors evaluated IMZ toxicity on the zebrafish model, highlighting an increment in
Fusobacteria and
Firmicutes and a decrement in
Proteobacteria and
Bacteroidetes after exposure to 1 mg/L IMZ for 21 days
[86]. Even propamocarb (PM) showed significant alteration in the GM of zebrafish after 7 days of exposure to 1 mg/L. At the phylum level,
Proteobacteria,
Bacteroidetes and
Firmicutes were increased
[87]. PM has been shown to be able to cause alterations in the GM of mice exposed to 3, 30, and 300 mg/L for 28 days. At the phylum level, the abundance of
Firmicutes,
Proteobacteria,
Saccharibacteria,
Actinobacteria and
Tenericutes were reduced, while
Bacteroidetes,
Acidobacteria,
Chlorobacteria and
Planctomycetes showed a growth
[88]. In chickens, thiram exposure was shown to significantly alter the composition of the gut microbiota, affecting the relative abundance of
Firmicutes and
Proteobacteria. In addition, liver histopathological structure changes were observed, accompanied by the increased blood lipid parameters
[89]. Wang et al. investigated the toxicity of two fungicides, chlorothalonil and procymidone, in mice. Both of the compounds impaired intestinal barrier function linked to glucolipid metabolism. Moreover, the histopathological analysis showed liver damage. Lastly, gut microbiota in the colon contents was significantly affected, with an increment in the
Firmicutes-to-
Bacteroides ratio
[90]. See .
6. Pesticide Mixtures
Lukowicz et al. aimed to assess the toxicity of a mixture of six pesticides (boscalid, captan, chlorpyrifos, thiophanate, thiacloprid, and ziram) in mice, suggesting that pesticide treatment could affect the regular gut microbiota composition and the host metabolic homeostasis
[91]. The perinatal exposure to a low-dose pesticide cocktail made up of six pesticides (chlorpyrifos, thiacloprid, thiophanate, captan, ziram and boscalid) administered to mice resulted in alterations in the urinary and faecal metabolite due to the alterations in the gut microbial communities
[92].
7. Future Perspectives
Until now, most of the studies evaluating the interaction between chemical agents and microbiota have limited themselves to observing qualitative or quantitative alterations in the composition of the GM. Even if more and more studies are going beyond this approach by introducing mechanistic hypotheses and evaluating them on experimental models, mostly in vitro, it is necessary to clarify these interactions further at the molecular level.
Moreover, the evaluation of GM-mediated effects is hard to define, since not all GM perturbations will develop a disorder in the host. Thus, biologically significant perturbation on humans should be identified. For this purpose, an integration of innovative biology techniques must be used to gain new details on the critical GM perturbations resulting in a disease. All this effort is needed to obtain useful tools for a valid risk assessment in populations exposed for environmental or occupational reasons, and possibly obtain the necessary elements for any modification of the regulatory policies and threshold limit value.