1000/1000
Hot
Most Recent

Age-related macular degeneration (AMD) is central vision loss with aging, was the fourth main cause of blindness in 2015, and has many risk factors, such as cataract surgery, cigarette smoking, family history, hypertension, obesity, long-term smart device usage, etc. In general, AMD drug candidates from natural products are more effective at treating early and intermediate AMD.

| Classification | Application Route and Therapeutic Effect | Reference | |
|---|---|---|---|
| Corticosteroids | Dexamethasone | 1. Topical application with artemisinin
|
[12] |
2. Three combined therapies: dexamethasone, an anti-VEGF drug, and verteporfin with photodynamic therapy
|
[13] | ||
| Triamcinolone Acetonide (TA) | 1. Intravitreal injection
|
[14] | |
2. Combined intravitreal TA and bevacizumab injection
|
[15] | ||
| Spironolactone (mineralocorticoid receptor antagonist) | Oral administration
|
[16] | |
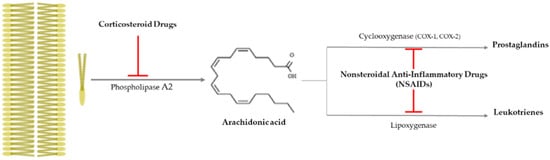
| NSAIDs | Aspirin | Topical application
|
[17][18][19] |
| Nepafenac (prodrug) | Topical application
|
[20] | |
| Diclofenac, Ketorolac | Intravitreal diclofenac and ketorolac injection
|
[21] | |
| Therapeutic Mechanism |
Natural Product | Application | References | ||||
|---|---|---|---|---|---|---|---|
| Species | Applied Characteristic | Effective Compound | Model | Route | Minimum Effective Dose |
||
| Inhibition of oxidative stress and apoptosis | Arctium lappa L. leaf | 100% EtOH extract | Phenolic and flavonoid | RPE cell | Media | 30 μg/mL for 24 h | [28] |
| Mouse | I.P. | 50 mg/kg for 4 w | |||||
| Eucommia ulmoides | Genipin (glycosidic ligand) |
Genipin (glycosidic ligand) |
ARPE-19 cell | Media | 30 μM for 24 h | [29] | |
| Fruit or Vegetable | Delphinidin (anthocyanidin) |
Delphinidin (anthocyanidin) |
ARPE-19 cell | Media | 25 μg/mL for 24 h | [31] | |
| Glycyrrhiza glabra L. root | Glabridin (isoflavonoid) |
Glabridin (isoflavonoid) |
RPE cell | Media | 2 μM for 2 h | [32] | |
| Mouse | I.P. | 20 mg/kg for 1 w | |||||
| Inhibition of inflammation and apoptosis | Scutellaria baicalensis Georgi root | 5,7-dihydroxy-8-methoxyflavone (wogonin) |
5,7-dihydroxy-8-methoxyflavone (wogonin) |
ARPE-19 cell | Media | 10 μM for 24 h | [33] |
| Inhibition of oxidative stress, inflammation, and apoptosis | Prunella vulgaris var. L | Water extract | Rosmarinic Acid | ARPE-19 cell | Media | 100 μg/mL for 24 h | [34] |
| Mouse | P.O. | 100 mg/kg for 4 day | |||||
| Inhibition of apoptosis | Vaccinium uliginosum L | Water extract | Polyphenol | ARPE-19 cell | Media | 100 μg/mL for 24 h | [35] |
| Inhibition of pyroptosis | Scutellaria baicalensis Georgi | Baicalin | Baicalin | ARPE-19 cell | Media | 50 μg/mL for 72 h | [36] |
| Inhibition of carbonyl stress | Lycopersicum esculentum L. (Tomato) |
n-hexane extract | β-carotene | ARPE-19 cell | Media | 1 μM-β-carotene for 24 h |
[38] |
| Inhibition of G2/M phase arrest | Fruit or Vegetable | Lutein | Lutein | ARPE-19 cell | Media | 25 μg/mL for 24 h | [27] |
| Inhibition of VEGF activation | Bile Acid (Animal) |
Taurocholic acid | Taurocholic acid | HRPEpiC cell | Media | 100 μM for 48 h | [41] |