1000/1000
Hot
Most Recent

Asphaltenes is the heaviest and most polar fraction of petroleum not yet wery well characterized and only presently define by they solubility.
Petroleum, as the most important source of energy and raw chemical materials, is a complex but delicately balanced system that depends on the relationship of its constituent fractions to each other [1]. Hence, the disturbance of these interactions, such as recovery and refining, may cause sediment formation and asphaltene deposition [2][3], which brings about many negative effects to the petroleum industry, such as the deactivation of catalysts, blocked pipelines, and deposition on the internal surface of the reservoirs [4][5][6][7][8].
Asphaltenes are the most polar fraction in crude oil, providing very low economic value and causing adverse effects to the oil industry. Images of n-heptane petroleum asphaltenes are shown in Figure 1. The content and characteristics of asphaltenes depend to a greater or lesser extent on the source of the crude oil [9]. Operationally, asphaltenes have up to now been defined as insoluble compounds in aliphatic hydrocarbons such as n-pentane or n-heptane, and soluble in aromatics such as toluene and benzene [10]. Asphaltenes are dark-brown-to-black friable solids that have no definite melting point, and usually foam and swell upon heating, leaving a carbonaceous residue [11]. The molecular weights of asphaltenes span a wide range, from hundreds to millions, leading to speculation about self-aggregation [12]. Carbon and hydrogen are the most abundant elements in asphaltenes, and the contents of carbon and hydrogen are usually greater than 90 wt%. These values correspond to a hydrogen-to-carbon atomic ratio of 1.15 in n-heptane (n-C7) asphaltenes [13]. In contrast to carbon and hydrogen, the content of undesired heteroatoms in asphaltenes usually greatly varies, especially sulfur [14]. Sulfur contents vary from 0.05 to 7.0 wt% [13]. On the other hand, the nitrogen content of asphaltene constituents has a somewhat lesser degree of variation (0.05–0.5 wt%), and oxygen contents generally less than 1.0 wt% [15]. In addition, there are some metallic elements in asphaltenes that are distributed in the range of 0–4000 ppm, among which nickel and vanadium are the most abundant. Metal atoms in asphaltenes are usually present in the form of metalloporphyrins [16], and as so-called “nonporphyrins”, which has not been proved [17].

Figure 1. Laboratory sample of asphaltenes extracted from crude oil in n-heptane (left) and n-pentane (right).
The composition and properties of asphaltenes have always been among the most important issues for petrochemistry. Over several decades, many researchers have contributed to the characterization, analysis, and determination of the physicochemical properties of asphaltenes with various analytical techniques, but these studies have only been partially successful, mainly because asphaltenes exhibit significant complexity. Asphaltene molecules are highly condensed and relatively high in undesired heteroatoms and metals, leading to stubborn self-aggregation [18]. Asphaltenes, defined as a solubility class, differ from a chemical class, so some variability among different asphaltenes is expected. To further complicate the problem, multifarious differently sourced crude oils and preparation procedures exist for asphaltenes. The characterization of asphaltenes is still a very difficult and challenging issue.
The precipitation and filtration of asphaltenes is generally carried out by using small molecules, n-alkanes. The procedure is performed with a variety of standard methods, as shown in Table 1. Each provides different results [19][20].
Table 1. Variety of asphaltene precipitation methods.
| Method | Solvent | Solvent/Oil Ratio | Operating Conditions | Filter Media |
|---|---|---|---|---|
| ASTM D-3279-07 | n-heptane | 100:1 | Reflux for 30 min, settle at ambient for 1 h, filter at 38–49 °C | Fiberglass, 1.5 μm |
| ASTM D-4124-01 | n-heptane | 100:1 | Heat on steam bath for 30 min, settle at ambient overnight | Slow/medium paper, ∼10 μm |
| ASTM D-4124-09 | iso-octane | 100:1 | Reflux for 2 h, settle at ambient for 2 h | Medium glass frit, 10 μm |
| WRI | n-heptane | 40:1 | Heat to 80 °C for 5 min, stir at ambient for 16 h, settle for 30 min | Medium glass frit, 10 μm |
| ASTM D-6560-00 | n-heptane | 30:1 | Reflux for 60 min, settle at ambient for 90–150 min | Whatman 42 paper, 2.5 μm |
| ASTM D-2007-03 | n-pentane | 10:1 | Settle at ambient for 30 min | Rapid paper, 20 μm |
| IFP 9313 absorbance versus maltenes at 750 nm | n-heptane | 20:1–200:1 | Heat to 80 °C for 5 min, filter at ambient | Cellulose ester filter, 0.45 μm |
Asphaltenes, as the most mysterious fraction in petroleum, have had their properties studied for decades. The bulk properties of asphaltenes, such as element content, density, and thermogravimetric analysis, are nowadays well-known and can be readily determined via correlative analytical instruments [21][22]. A more comprehensive understanding of asphaltenes requires the utilization of specialized analysis methods. For example, Nuclear Magnetic Resonance (NMR) [23][24] and X-ray diffraction (XRD) [25] can be used to determine the average molecular parameters of asphaltenes. Vapor pressure osmometry (VPO), size exclusion chromatography (SEC), and mass spectrometry (MS) techniques can be used to measure the molecular weight distribution of asphaltenes [26][27][28][29].
In recent years, with its continuous development, MS technology and especially ultra-high-resolution (UHR) mass spectrometers are an indispensable tool for analyzing the chemical properties and molecular composition of asphaltenes [30][31][32]. Electrospray ionization (ESI) equipped Fourier transform ion cyclotron resonance–mass spectrometry (FT-ICR–MS) has long been used to characterize the molecular composition of asphaltenes, as early as a decade ago. ESI preferentially ionizes heteroatom-containing compounds such as polar N-, S-, O-, and metal-containing species. ESI FT-ICR–MS has been widely used to analyze the molecular composition of heteroatomic compounds in asphaltenes and their corresponding saturates, aromatics, and resins [33][34]. In addition, it is widely used in the research of petroleum porphyrin and geochemical porphyrin due to the good response of metalloporphyrins in ESI [35][36]. Compared to ESI, atmospheric-pressure photoionization (APPI) ionizes a wider range of compounds, including nonpolar compounds [37]. The APPI ionization source may obtain asphaltene-composition information not seen by ESI, such as polycyclic aromatic hydrocarbons (PAHs) [38]. Thus, APPI is widely used to characterize the molecular composition of petroleum asphaltenes and other components [39][40]. Although the resolution of Orbitrap is not as high as that of FT-ICR, its resolution ability cannot be ignored, and its stronger isolation and collision capabilities have unique advantages in studying the molecular composition and structural information of asphaltenes [41]. In addition, other ionization techniques (e.g., APCI, APLI, LDI, MALDI, and ASAP) equipped with ultra-high-resolution mass spectrometers are also applied to characterize asphaltenes [42][43][44][45][46].
Ultra-high-resolution mass spectrometers equipped with multiple ionization sources have an absolute advantage in the qualitative research of asphaltenes’ molecular composition, but their quantitative results are affected by differences in ionization response and other substrates. Due to the limitations of molecular MS for the direct and quantitative identification of asphaltene compounds, inductively coupled plasma–high-resolution mass spectrometry (ICP–HR-MS) is an efficient tool for studying the size distribution of vanadium-, nickel-, and sulfur-containing compounds in asphaltenes. When ICP–MS is used to study asphaltenes, liquid chromatography (LC) is often used for online separation, and gel-permeation chromatography (GPC) is the most commonly used separation principle. The asphaltene solution (usually dissolved in tetrahydrofuran) is separated into high- (HMW), medium- (MMW), and low-molecular-weight (LMW) fractions after passing through GPC; then, it is analyzed online by mass spectrometry and simultaneously detected by UV or other detectors [47]. Despite criticisms of GPC, coupling GPC with ICP–MS allows for the identification and quantification of relative sizes associated with various V, Ni, and S compounds in asphaltenes [48]. Reinjected experiments revealed that the dissociation of asphaltene aggregates and reaggregation of LMW fractions occur after the isolation, as shown in Figure 2 [47].
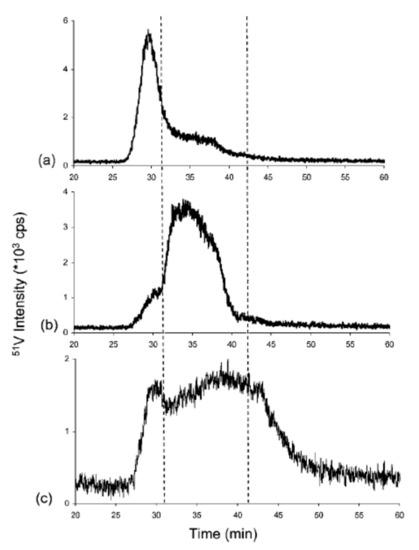
Figure 2. Chromatograms obtained by gel-permeation chromatography (GPC) inductively coupled plasma (ICP) MS of collected and reinjected (a) high-molecular-weight (HMW), (b) medium-molecular-weight (MMW), and (c) low-molecular-weight (LMW) fractions [47].
Recently, high-resolution scanning probe microscopy has emerged as effective method for elucidating molecular structures, offering the unique capability of imaging a single adsorbate on the atomic scale. Attempts were made to use scanning tunneling microscopy (STM) to characterize asphaltenes, but no atomic resolution could be achieved on asphaltene molecules [49]. The latest advances in atomic force microscopy (AFM) enabled the direct observation of individual asphaltene molecules. Schuler et al. [50] studied more than 100 asphaltene molecules using AFM and STM, and provided the direct measurement of the tremendous range of molecular structures in asphaltenes. Images of asphaltene molecules derived from coal and petroleum are shown in Figure 3. This work is groundbreaking in the history of asphaltenes, allowing for us to have a direct visual understanding of the structure of some molecules in asphaltenes. This technique is also used to identify colloidal particles associated with asphaltene aggregates present in crude oils and the model system [51]. AFM even allows for direct observations on the specific structure of some biomarkers, such as the characterization of substitution patterns on petroporphyrins [52]. This technology constitutes a paradigm shift in the analysis of complex molecular mixtures, and may be applied to molecular electronics, organic light-emitting diodes, and photovoltaic devices [50].
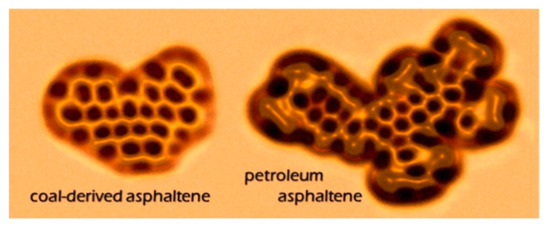
Figure 3. Atomic force microscopy (AFM) images of coal-derived asphaltenes and petroleum asphaltenes [50].
Petroleum is a complex mixture containing not only carbon, hydrogen, sulfur, oxygen, and nitrogen, but also many trace elements, including 45 metals, such as V, Ni, and Fe [53]. It is well-known that a fair amount of metal in crude oil exists in the asphaltene fraction. These metals are detrimental to petroleum processing, especially for accelerating catalyst deactivation [54]. Metal content is one of the important parameters for evaluating crude oil. Modern instrumental approaches to determine trace metals and asphaltenes in crude oil is by means of physical methods. Analytical methods were developed for the determination of metals in crude oils and derivatives using various analytical techniques, including flame atomic absorption spectrometry (FAAS) [55], graphite furnace atomic absorption spectrometry (GFAAS) [56], inductively coupled plasma optical-emission spectroscopy (ICP–OES) [57], inductively coupled plasma–mass spectrometry (ICP–MS) [58], X-ray fluorescence spectroscopy [59], spectrophotometry [60], and high-performance liquid chromatography (HPLC) [61]. To date, concentrations of these metals in petroleum have mostly been determined by ICP–OES and ICP–MS because of their distinguished sensitivity, repeatability, and operability [62]. A large amount of data indicates that metal content in crude oil is between 1 and 10,000 μg/g, and concentrations of nickel and vanadium can reach up to several thousand ppm (w/w) [63]. The concentration of these metals in the asphaltene fraction is the most abundant. The concentration range of V, Ni, and Fe in asphaltenes from different studies is shown in Table 2.
Table 2. Concentrations of V, Ni, and Fe in asphaltenes with different origins.
| Asphaltene Origin | Concentration Range of V (ppm) | Concentration Range of Ni (ppm) | Concentration Range of Fe (ppm) | Reference |
|---|---|---|---|---|
| Venezuela crude oil | 1300–4000 | 300–410 | [64] | |
| Kuwait crude oil | 200–800 | 50–120 | [65] | |
| Athabasca oil sand | 640 | 240 | 260 | [66] |
| Utah oil sand | 21 | 170 | 4820 | [66] |
| Russia Tatarstan crude oil | 200–10,000 | 120–550 | [67] | |
| China Qingchuan gilsonite | 3888 | 366 | 491 | [68] |
| Texas shale | 270 | 257 | 634 | [36] |