1000/1000
Hot
Most Recent

Cancer cells frequently overexpress specific surface receptors providing tumor growth and survival which can be used for precise therapy. Targeting cancer cell receptors with protein toxins is an attractive approach widely used in contemporary experimental oncology and preclinical studies.
Cancer treatment has traditionally been based on surgery, radiation, and chemotherapy, which have shown limited therapeutic benefits in patients with metastatic disease. Despite significant advances in the development of systemic treatment, traditional chemotherapeutic agents cause serious side toxicity, restricting treatment to certain therapeutic dosages. In light of this, new approaches to selective treatment are urgently needed.
Protein toxins possessing such features as high cytotoxicity and efficiency have become promising components for anticancer therapy. Cancer cells frequently upregulate surface receptors that promote growth and survival, that is why various antigen-specific proteins including antibodies, antibody fragments (e.g., Fab and scFv), and other protein scaffolds (e.g., affibody and DARPin) have been developed as a moiety to target cancer cells [1][2]. Being genetically encoded, toxins can be expressed as fusion proteins with targeting moieties mentioned above and can have a wide range of modifications to prolong circulation in the bloodstream and increase tumor retention. Complete biodegradation within an organism is also an important advantage of protein toxins as anticancer agents [3][4].
In addition to natural protein toxins, designed toxins are also used in experimental oncology, for example, as an alternative to chemical photosensitizers [5][6][7][8]. The main advantage of protein photosensitizers is the opportunity to use a genetic engineering approach to combine cytotoxic and targeting moieties, avoiding chemical conjugation.
The history of targeted toxins began with the chemical conjugation of natural diphtheria toxin (DT) with anti-lymphocyte antibodies or their F(ab)2 fragments to produce agents for killing lymphoblastoid tumor cells [9]. This strategy helped to couple cell-specific delivery of antibodies with extremely high toxicity of DT, previously shown for mammalian cells [10]. The first generation of immunotoxins used chemical conjugation to couple natural toxins with full-length antibodies [11]. The introduction of hybridoma technology [12] enabled the production of precisely characterized bifunctional agents with a certain specificity. The second generation of immunotoxins arise due to the use of truncated fragments of protein toxins, lacking natural tropism, which helped to reduce in vivo side toxicity [13]. Over time, the variety of toxins used in the design of targeted therapy has grown [14][15], but the next breakthrough was made due to molecular cloning, which allowed for the production of the third-generation immunotoxins: fusion proteins consisting of antibody fragments linked to enzymatically active toxin domains [16][17].
Soluble targeted toxins are thought to be the embodiment of a “magic bullet” idea. Being applied systemically, these agents can reach disseminated, metastatic, or inoperable tumors and kill cancer cells. Still, there are several factors affecting the efficiency of targeted toxins (summarized in Figure 1).
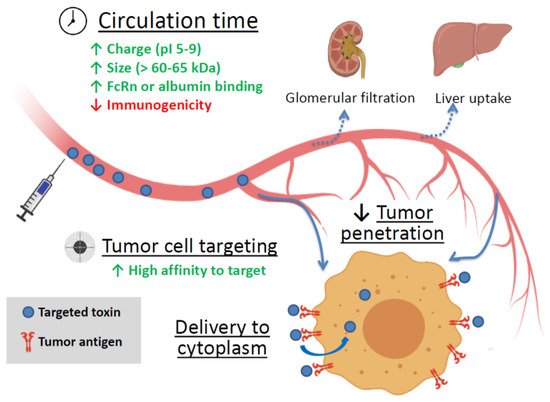
Figure 1. The main factors affecting the efficiency of targeted toxin. Green up arrows—factors enhancing circulation time and tumor cell targeting. Red down arrow—reducing factor. FcRn is the neonatal Fc receptor.
Despite the successful use of immunotoxins, immunotherapy strategies are still expensive, mainly due to the complicated preparation process. Immunotoxins can also stimulate the host immune system and trigger the production of neutralizing antibodies. Intravenous administration of targeted protein toxins may be characterized by poor pharmacokinetic profiles in addition to non-specific distribution in tissues and organs of the body and can cause serious side effects including systemic toxicity. Besides, the penetration of anticancer drugs into tumor tissues is usually low and the high doses of drugs are required for treatment [18][19]. The use of nanocarriers, especially the targeted ones, for delivering toxins to tumor foci may improve the pharmacokinetics and pharmacodynamics of agents, control drug release, improve the specificity, increase internalization and intracellular delivery, and reduce systemic toxicity [20]. Nanocarriers can facilitate selective accumulation in tumors via the enhanced permeability and retention (EPR) effect and active cellular uptake [21]. Among various nanoscale drug carriers, liposomes, polymeric nanoparticles and noble metal nanoparticles have demonstrated the greatest potential in clinical application [22][23][24].
Liposomes have proven to be an efficient vehicle for delivering a high molecular weight neurotoxin botulinum toxin A to treat hypersensitive bladder and overactive bladder (OAB) without systemic injection [25].
Recently, a new method has been proposed for the preparation of small (80–90 nm) unilamellar antigen-targeted liposomes containing large amounts (thousands of protein molecules per liposome) of highly toxic PE40 [26] (Figure 2a). Efficient encapsulation of the proteins was achieved through electrostatic interaction between positively charged toxin proteins at pH lower than pI and negatively charged liposome membrane. The external surface of proteoliposomes were functionalized with covalently coupled DARPin_9-29 using “click chemistry” through a relatively long flexible linker. Functionalized proteoliposomes specifically bind to HER2-positive cells and after internalization cause cell death at subnanomolar concentrations [27].
Figure 2. (a) Small unilamellar targeted liposomes containing PE40 [26]; (b) Hybrid biofunctional nanocomplex based on radioactive 90Y bearing core-shell UCNP and functionalized with targeted toxin DARPin-PE40 [28].
The killing mechanisms of protein toxins can vary, but they differ from the mechanisms that are implemented in conventional chemotherapy [4], so an obtained resistance to chemotherapeutic agents does not affect the effectiveness of protein toxins. Furthermore, the mechanism complementation can provide a synergistic effect of combined therapy. In addition, protein toxins are not mutagens and should not accelerate tumor progression due to enhanced mutagenesis. They can be mass-produced cheaply in bacteria as homogeneous proteins [16].
Toxins of bacterial and plant origin commonly used as cytotoxic component in chimeric proteins in anticancer therapy are summarized in Table 1. The most toxic proteins include enzymes that inhibit translation at the elongation step. Unsurprisingly, most of them arise from natural toxins that have been effectively preselected by evolution.
| Mechanism of Action | Details | Examples | References |
|---|---|---|---|
| eEF2 inactivation | ADP-ribosylates elongation factor 2 (eEF2) and halt protein synthesis at the elongation step | Pseudomonas exotoxin A (PE, ETA) | [29][30] |
| Diphtheria toxin (DT) | [9][31] | ||
| Ribosome inactivation | N-glycosidase depurinates a critical adenine in 28S rRNA, which results in the inability of the ribosome to bind elongation factor 2, thereby blocking protein translation | Ricin | [32][33][34] |
| Shiga toxin (Stx) | [35] | ||
| Abrin | [36][37][38] | ||
| RNA degradation | Nonspecific RNA cleavage blocks protein synthesis and leads to apoptosis | Barnase | [39][40] |
| Binase | [41] | ||
| Cell signaling disruption | The cleavages of the MAP kinase family members leading to their inactivation; uncontrolled conversion of ATP to cAMP | Anthrax toxin | [42] |
| Photoinduced ROS production | The proteins absorb exciting light and produce reactive oxygen species | KillerRed | [43][44] |
| miniSOG | [45] | ||
| Direct apoptosis induction | Effector caspases cleavage | Granzyme B | [46] |
| Enhanced diffusion of anticancer drug | Vascular network modulation | Botulinum neurotoxin | [47][48] |
| Pore formation for better intracellular delivery | Listeriolysin O | [49][50] | |
| Streptolysin-O | [51][52] |
The protein toxins high toxicity is one of main advantages of these molecules but at the same time it increases the risk and severity of side effects. The side toxicity of a protein can be based on a direct cell killing and inflammation induction [53]. The most common side effects caused by DT, PE, and ricin include vascular leak syndrome, hepatotoxicity, and kidney damage [54][55][56]. In addition, Shiga toxin is notorious for its ability to cause hemolytic uremic syndrome (HUS), potentially leading to life-threatening complications [57][58]. The production of neutralizing antibodies can also serve as a cause on side effects due to anaphylaxis reactions.
To date the number of strategies were developed to reduce protein drug off-target toxicity, the main tools are summarized in Table 2.
| Strategy Used for Side Toxicity Reduction | Principle | References |
|---|---|---|
| Impairment of natural tropism | Removing the natural targeting domains of AB toxins | [59] |
| Introduction of point mutations attenuating the target binding | [60] | |
| Construction of miniaturized toxin variants | Deletion of protein parts not directly involved in toxin mechanism of action to reduce any non-specific interaction and immunogenicity | [61][62][63] |
| Tumor-specific activation of a toxin | The replacement of furin cleavage site to tumor-specific proteases cleavage sites (MMP, uPA) | [64][65][66] |
| RES cells inactivation | Macrophages blockade decreasing toxic nanoparticles uptake | [67][68] |
The use of several toxic mechanisms or several target molecules makes it possible to compensate for the deficiencies of effector molecules, increase their efficiency and avoid selection of resistant cells. The designed toxic proteins capable of ROS production and fused to UCNP or luciferase make it possible to overcome the shallow depth of excitation light penetration, thus providing a novel approach to PDT of deeply located tumors.
Despite the numerous breakthrough solutions in cancer treatment, the problem is still far from being solved. It is worth mentioning that only two toxin-based molecules, namely Diphtheria toxin-based DAB389IL2 and DAB389IL3 [89][90], have been approved in late-stage clinical evaluation. Recently, a PE-based immunotoxin Moxetumomab Pasudotox (Lumoxiti), targeting CD22, has been approved for the treatment of patients with hairy cell leukemia [62]. In the future, new targeted therapies and combinations with increased selective anticancer activity and minimal side effects will be studied, which will increase the clinical efficacy of patients with various types of cancer.